Adjusting the Coordination Configuration by Changing Electrostatic Potential: Introducing N/O/S Heteroatoms Based on the Electronic Effect
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
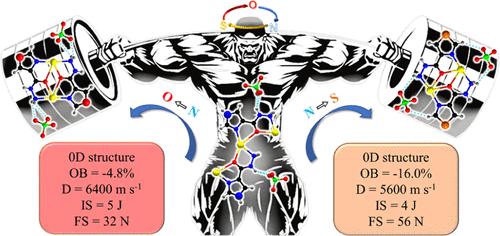
Energetic coordination compounds (ECCs) have demonstrated unique advantages in regulating the physicochemical properties of energetic materials through the design of organic ligands. The fundamental approach involves altering the electron cloud density distribution of organic ligands to modify the characteristics of coordination sites and, thus, achieve new coordination configurations. In this study, Mulliken charge distribution and surface electrostatic potential analysis were used to elucidate the effects of pyridinic N, pyrrolic N, oxazolic O, and thiazolic S on the electron cloud density of carbohydrazide groups through the induction effect and conjugate effect. Furthermore, three AgClO4-based ECCs were synthesized based on 1H-imidazole-4-carbohydrazide, oxazole-4-carbohydrazide, and thiazole-4-carbohydrazide. Single-crystal X-ray diffraction analysis revealed that [Ag(IZ-4-CA)ClO4]n has a one-dimensional (1D) chain structure, while Ag2(OZCA)2(ClO4)2 and Ag2(SZCA)2(ClO4)2 exhibit zero-dimensional structures. The 1D structure, with good planarity, results in [Ag(IZ-4-CA)ClO4]n having lower mechanical sensitivity (IS = 21 J, FS = 80 N). The introduction of oxazolic O enhances oxygen balance (OB), leading to a higher predicted detonation velocity and pressure for Ag2(OZCA)2(ClO4)2 (D = 6.4 km s–1, P = 23.6 GPa). Although the introduction of thiazolic S is unfavorable for improving oxygen balance, Ag2(SZCA)2(ClO4)2 exhibits the highest initial decomposition temperature among the three, at 232 °C. Additionally, initiation tests demonstrated that three ECCs can successfully detonate cyclotrimethylenetrinitramine (RDX), indicating good initiation capabilities.
通过改变静电势调整配位构型:基于电子效应引入 N/O/S 杂原子
高能配位化合物(ECC)在通过设计有机配体来调节高能材料的物理化学特性方面具有独特的优势。其基本方法是通过改变有机配体的电子云密度分布来改变配位位点的特性,从而实现新的配位构型。本研究利用 Mulliken 电荷分布和表面静电势分析,通过诱导效应和共轭效应,阐明了吡啶 N、吡咯烷 N、噁唑 O 和噻唑 S 对羧酰肼基团电子云密度的影响。此外,还以 1H-咪唑-4-羧酰肼、噁唑-4-羧酰肼和噻唑-4-羧酰肼为基础,合成了三种基于 AgClO4 的 ECC。单晶 X 射线衍射分析表明,[Ag(IZ-4-CA)ClO4]n 具有一维(1D)链结构,而 Ag2(OZCA)2(ClO4)2 和 Ag2(SZCA)2(ClO4)2 则呈现零维结构。一维结构具有良好的平面性,因此[Ag(IZ-4-CA)ClO4]n 的机械灵敏度较低(IS = 21 J,FS = 80 N)。引入噁唑 O 可提高氧平衡 (OB),从而提高 Ag2(OZCA)2(ClO4)2 的预测引爆速度和压力(D = 6.4 km s-1,P = 23.6 GPa)。虽然噻唑 S 的引入不利于改善氧平衡,但 Ag2(SZCA)2(ClO4)2 的初始分解温度在三者中最高,为 232 ℃。此外,起爆试验表明,三种 ECC 均能成功引爆环三亚甲基三硝胺(RDX),这表明它们具有良好的起爆能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


