Anionic polymers formed by dinuclear rhodium units and dicyanide silver/gold moieties
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
The synthesis, characterization and structural determination of four complexes with formula (PPh4)n{[Rh2(μ-O2CPh)4][Au(CN)2]}n (1) and Kn{[Rh2(μ-O2CR)4][M(CN)2]}n (M = Au, R ![]() CH2OEt (2), CH2OMe (3); M = Ag, R
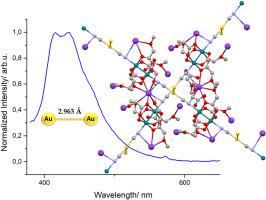
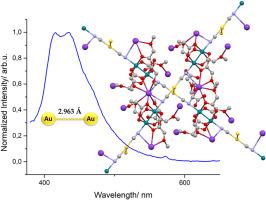
CH2OEt (2), CH2OMe (3); M = Ag, R ![]() CH2OMe (4)) are described. In all cases, the [CN-M-CN]– (M = Au, Ag) group is bridging the axial positions of the dirhodium units giving one-dimensional chains. The packing of the chains in the solid state depends on the nature of the carboxylate group of the dimetallic unit, the counterion present in the complex and the metal itself. The most striking change is observed in the Kn{[Rh2(μ-O2CCH2OMe)4][Au(CN)2]}n complex (3), where the chains are positioned perpendicular to each other with an Au⋯Au interchain distance of 2.963 Å. The emission studies of this complex upon excitation at 350 nm show a clear broad emission band centered at 430 nm, while no luminescence is observed for the other compounds. The appearance of emission properties only in the case of complex 3 can be explained by its short aurophilic interactions.
CH2OMe (4)) are described. In all cases, the [CN-M-CN]– (M = Au, Ag) group is bridging the axial positions of the dirhodium units giving one-dimensional chains. The packing of the chains in the solid state depends on the nature of the carboxylate group of the dimetallic unit, the counterion present in the complex and the metal itself. The most striking change is observed in the Kn{[Rh2(μ-O2CCH2OMe)4][Au(CN)2]}n complex (3), where the chains are positioned perpendicular to each other with an Au⋯Au interchain distance of 2.963 Å. The emission studies of this complex upon excitation at 350 nm show a clear broad emission band centered at 430 nm, while no luminescence is observed for the other compounds. The appearance of emission properties only in the case of complex 3 can be explained by its short aurophilic interactions.
由双核铑单元和双氰基银/金分子形成的阴离子聚合物
本文介绍了四种络合物的合成、表征和结构测定,它们的化学式分别为 (PPh4)n{[Rh2(μ-O2CPh)4][Au(CN)2]}n (1) 和 Kn{[Rh2(μ-O2CR)4][M(CN)2]}n (M = Au, R = CH2OEt (2), CH2OMe (3); M = Ag, R = CH2OMe (4))。在所有情况下,[CN-M-CN]-(M = Au、Ag)基团都桥接在二铑单元的轴向位置,形成一维链。固态链的堆积取决于二金属单元的羧酸基团、配合物中的反离子和金属本身的性质。在 Kn{[Rh2(μ-O2CCH2OMe)4][Au(CN)2]}n(3)复合物中观察到了最显著的变化,其中链的位置相互垂直,Au--Au 链间距离为 2.963 Å。只有复合物 3 具有发射特性,这可以用它的短亲欧相互作用来解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


