Modulating Fe sites by La in porous MnFe2O4 for enhanced removal of ROX: Synergy of efficient adsorption and PMS activation
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
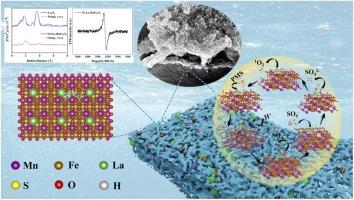
Catalytic-adsorption method is a promising strategy for degrading organoarsenic compounds and removing secondary inorganic arsenic. The method relies significantly on heterogeneous catalysts with selectively adsorption and enhanced peroxymonosulfate (PMS) activation capacity. In this study, active sites for selective adsorption and PMS activations were developed by modulating the Fe-sites in porous MnFe2O4 through La-doping. Synchrotron radiation, EPR, and XPS characterizations confirmed the presence of oxygen vacancies, metal hydroxyl groups ![]() M(Fe/Mn/La)-(OH) and the active Fe(II)/Mn(II,III), as well as the fine structure of La occupied sites. Theoretical calculations indicate that the generation of Vo would increase the local electron cloud density of La dopants, leading to the transfer of local electrons into the bulk phase. The electron transfer characteristics result in the raising the d-band center of MnFe2O4 and lowering the Gibbs free energy of the intermediate state, thus promoting 1O2 generation. In 3% La-MnFe2O4/PMS system, 96% ROX (10 mg/L) were removed within 35 min with the secondary inorganic arsenic levels below 10 μg/L. The rate coefficients k for ROX removal in porous 3%La-MnFe2O4/PMS is 4.05 times higher than that in MnFe2O4/PMS. ROX was effectively removed in different water matrices (Liao River, Hun River, and groundwater), demonstrating the practical application potential of 3%La-MnFe2O4/PMS system. Under continuous flow conditions, the average of 97.9% and 87.3% of ROX were removed from ultrapure water and groundwater, respectively, over a 10-hour continuous run. This study highlights the high-performance spinel La-MnFe2O4 for the synergistic enhancement of PMS activation, secondary arsenic adsorption, and improved mass transfer, contributing to green and safe water treatment strategies.
M(Fe/Mn/La)-(OH) and the active Fe(II)/Mn(II,III), as well as the fine structure of La occupied sites. Theoretical calculations indicate that the generation of Vo would increase the local electron cloud density of La dopants, leading to the transfer of local electrons into the bulk phase. The electron transfer characteristics result in the raising the d-band center of MnFe2O4 and lowering the Gibbs free energy of the intermediate state, thus promoting 1O2 generation. In 3% La-MnFe2O4/PMS system, 96% ROX (10 mg/L) were removed within 35 min with the secondary inorganic arsenic levels below 10 μg/L. The rate coefficients k for ROX removal in porous 3%La-MnFe2O4/PMS is 4.05 times higher than that in MnFe2O4/PMS. ROX was effectively removed in different water matrices (Liao River, Hun River, and groundwater), demonstrating the practical application potential of 3%La-MnFe2O4/PMS system. Under continuous flow conditions, the average of 97.9% and 87.3% of ROX were removed from ultrapure water and groundwater, respectively, over a 10-hour continuous run. This study highlights the high-performance spinel La-MnFe2O4 for the synergistic enhancement of PMS activation, secondary arsenic adsorption, and improved mass transfer, contributing to green and safe water treatment strategies.
用 La 调节多孔 MnFe2O4 中的铁位点以提高 ROX 的去除率:高效吸附与 PMS 活化的协同作用
催化吸附法是一种很有前途的降解有机砷化合物和去除二次无机砷的方法。该方法在很大程度上依赖于具有选择性吸附和增强过一硫酸盐(PMS)活化能力的异相催化剂。在本研究中,通过掺杂 La 来调节多孔 MnFe2O4 中的 Fe 位点,开发出了选择性吸附和 PMS 活化的活性位点。同步辐射、EPR 和 XPS 表征证实了氧空位、金属羟基 M(Fe/Mn/La)-(OH)和活性 Fe(II)/Mn(II,III)的存在,以及 La 占位的精细结构。理论计算表明,Vo 的产生会增加 La 掺杂剂的局部电子云密度,导致局部电子转移到体相。电子转移特性会提高 MnFe2O4 的 d 带中心,降低中间状态的吉布斯自由能,从而促进 1O2 的生成。在 3% 的 La-MnFe2O4/PMS 系统中,在 35 分钟内可去除 96% 的 ROX(10 mg/L),二次无机砷含量低于 10 μg/L。多孔 3%La-MnFe2O4/PMS 系统去除 ROX 的速率系数 k 是 MnFe2O4/PMS 系统的 4.05 倍。在不同的水基质(辽河、浑河和地下水)中,ROX 都得到了有效去除,证明了 3%La-MnFe2O4/PMS 系统的实际应用潜力。在连续流动条件下,连续运行 10 小时,超纯水和地下水中的 ROX 平均去除率分别为 97.9% 和 87.3%。这项研究强调了高性能尖晶石 La-MnFe2O4 在协同增强 PMS 活化、二次砷吸附和改善传质方面的作用,有助于实现绿色安全的水处理策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


