In situ production of hydrogen peroxide from Fe, Mo co-doped N@TiO2 for organic pollutant degradation
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Degradation of organic pollutants by in-situ electrochemically synthetic hydrogen peroxide (H2O2) in electro-Fenton (EF) system is important for the treatment of wastewater. In this work, a Fe, Mo co-doped N@TiO2 electrocatalyst was constructed for H2O2 generation and applied to the degradation of organic dyes. The FeMoN@TiO2 catalyst in 0.1 M KOH exhibits excellent 2e- Oxygen reduction reaction (ORR) activity and stability, with a yield of up to 1409 mmol gcat-1h−1 and Faraday efficiency (FE) of 83.4 % at −0.7 V (relative to SCE) for more than 10 h. The in situ generated H2O2 delivers benign organic dyes degradation ability with high methylene blue (MB) removal rate of 98.2 % after 2 h in 0.1 M KOH without adding Fe2+. After adding Fe2+, the EF process occurs to degrade dyes more efficiently, with Congo red and phenol removal rates of more than 99.0 % and Ciprofloxacin (CIP) removal rate of more than 96.0 % in 0.1 M Na2SO4 solution. It is found that Mo doping increases the active sites of the catalyst, while the dopant of Fe can enhance can improve the electron transfer rate between the catalyst and reactants. Additionally, the synergistic Fe and Mo modulate the electronic structure of Ti, increasing the concentrations of Ti3+ and chemiadsorbed O. This enhances the electronic conductivity and the reactivity of the catalyst, leading to improved ability for H2O2 generation and organic pollutants degradation. Radical quenching experiments show that the in-situ generated H2O2 reacts with added Fe2+ to produce a large amount of ·OH, which is the main active substance for organic pollutants degradation. This study provides new insights for non-precious metal oxides to replace precious metals towards in-situ production of H2O2 and degradation of pollutants.
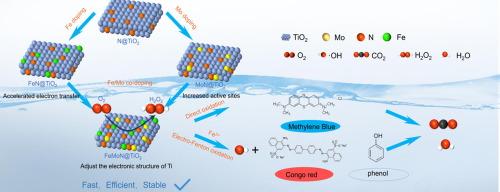
利用铁、钼共掺杂的 N@TiO2 原位制备过氧化氢以降解有机污染物
在电-芬顿(EF)系统中通过原位电化学合成过氧化氢(H2O2)降解有机污染物对废水处理非常重要。本研究构建了一种铁、钼共掺杂的 N@TiO2 电催化剂,用于生成 H2O2 并将其应用于有机染料的降解。在 0.1 M KOH 中,FeMoN@TiO2 催化剂表现出优异的 2e 氧还原反应(ORR)活性和稳定性,在 -0.7 V(相对于 SCE)电压下超过 10 小时,产率高达 1409 mmol gcat-1h-1,法拉第效率(FE)为 83.4%。原位生成的 H2O2 具有良性有机染料降解能力,在不添加 Fe2+ 的情况下,在 0.1 M KOH 中 2 小时后,亚甲基蓝(MB)的去除率高达 98.2%。在 0.1 M Na2SO4 溶液中,加入 Fe2+ 后,EF 过程会更有效地降解染料,刚果红和苯酚的去除率超过 99.0%,环丙沙星(CIP)的去除率超过 96.0%。研究发现,钼的掺杂增加了催化剂的活性位点,而铁的掺杂则提高了催化剂与反应物之间的电子转移率。此外,Fe 和 Mo 的协同作用调节了 Ti 的电子结构,增加了 Ti3+ 和化学吸附 O 的浓度,从而增强了催化剂的电子传导性和反应活性,提高了生成 H2O2 和降解有机污染物的能力。自由基淬灭实验表明,原位生成的 H2O2 会与添加的 Fe2+ 发生反应,产生大量 -OH,而 -OH 正是降解有机污染物的主要活性物质。这项研究为非贵金属氧化物取代贵金属在原位产生 H2O2 和降解污染物方面提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


