A temporal cortex cell atlas highlights gene expression dynamics during human brain maturation
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
The human brain undergoes protracted postnatal maturation, guided by dynamic changes in gene expression. Most studies exploring these processes have used bulk tissue analyses, which mask cell-type-specific gene expression dynamics. Here, using single-nucleus RNA sequencing on temporal lobe tissue, including samples of African ancestry, we build a joint pediatric and adult atlas of 75 cell subtypes, which we verify with spatial transcriptomics. We explore the differences between pediatric and adult cell subtypes, revealing the genes and pathways that change during brain maturation. Our results highlight excitatory neuron subtypes, including the LTK and FREM subtypes, that show elevated expression of genes associated with cognition and synaptic plasticity in pediatric tissue. The resources we present here improve our understanding of the brain during its development and contribute to global efforts to build an inclusive brain cell map. This Pediatric Cell Atlas study analyzes temporal cortex single-nucleus RNA sequencing datasets from eight diverse donors from 4 to 50 years of age, describing gene expression dynamics over the course of brain maturation.
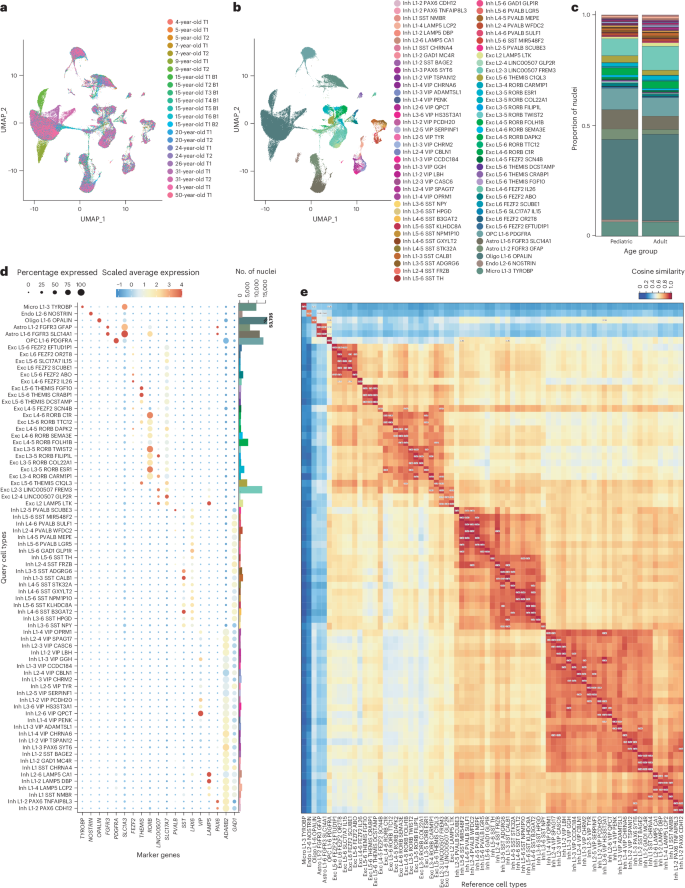

颞叶皮层细胞图谱突显人脑成熟过程中的基因表达动态
人脑在基因表达动态变化的引导下经历了漫长的产后成熟过程。大多数探索这些过程的研究都使用了大块组织分析,这掩盖了细胞类型特异性基因表达的动态变化。在这里,我们利用对颞叶组织(包括非洲血统样本)的单核 RNA 测序,建立了 75 种细胞亚型的儿科和成人联合图谱,并通过空间转录组学进行了验证。我们探索了小儿和成人细胞亚型之间的差异,揭示了大脑成熟过程中发生变化的基因和通路。我们的研究结果突显了兴奋性神经元亚型,包括 LTK 和 FREM 亚型,这些亚型在小儿组织中显示出与认知和突触可塑性相关基因的高表达。我们在此介绍的资源增进了我们对大脑发育过程的了解,并有助于全球构建包容性脑细胞图谱的努力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


