Photocatalytic low-temperature defluorination of PFASs
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Polyfluoroalkyl and perfluoroalkyl substances (PFASs) are found in many everyday consumer products, often because of their high thermal and chemical stabilities, as well as their hydrophobic and oleophobic properties1. However, the inert carbon–fluorine (C–F) bonds that give PFASs their properties also provide resistance to decomposition through defluorination, leading to long-term persistence in the environment, as well as in the human body, raising substantial safety and health concerns1–5. Despite recent advances in non-incineration approaches for the destruction of functionalized PFASs, processes for the recycling of perfluorocarbons (PFCs) as well as polymeric PFASs such as polytetrafluoroethylene (PTFE) are limited to methods that use either elevated temperatures or strong reducing reagents. Here we report the defluorination of PFASs with a highly twisted carbazole-cored super-photoreductant KQGZ. A series of PFASs could be defluorinated photocatalytically at 40–60 °C. PTFE gave amorphous carbon and fluoride salts as the major products. Oligomeric PFASs such as PFCs, perfluorooctane sulfonic acid (PFOS), polyfluorooctanoic acid (PFOA) and derivatives give carbonate, formate, oxalate and trifluoroacetate as the defluorinated products. This allows for the recycling of fluorine in PFASs as inorganic fluoride salt. The mechanistic investigation reveals the difference in reaction behaviour and product components for PTFE and oligomeric PFASs. This work opens a window for the low-temperature photoreductive defluorination of the ‘forever chemicals’ PFASs, especially for PTFE, as well as the discovery of new super-photoreductants. Photocatalysis at 40–60 °C is shown to be able to defluorinate perfluoroalkyl substances, known as ‘forever chemicals’, allowing the recycling of fluorine in polyfluoroalkyl and perfluoroalkyl substances as inorganic fluoride salt.
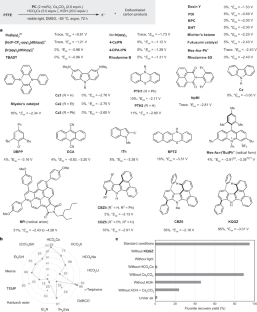

光催化低温脱氟处理全氟辛烷磺酸
聚氟烷基和全氟烷基物质(PFASs)通常具有较高的热稳定性和化学稳定性,以及疏水和疏油特性,因此在许多日常消费品中都能发现PFASs的身影1。然而,赋予 PFAS 特性的惰性碳-氟(C-F)键也使其不易通过脱氟分解,从而导致其在环境和人体中长期存在,引发了大量的安全和健康问题1,2,3,4,5。尽管最近在采用非焚烧方法销毁功能化全氟辛烷磺酸方面取得了进展,但全氟碳化物(PFCs)以及聚四氟乙烯(PTFE)等聚合全氟辛烷磺酸的回收工艺仍局限于使用高温或强还原试剂的方法。在此,我们报告了使用高度扭曲的咔唑芯超级光诱导剂 KQGZ 对 PFAS 进行脱氟的情况。一系列 PFAS 可在 40-60 °C的光催化条件下脱氟化。聚四氟乙烯的主要产物是无定形碳和氟化盐。全氟碳化物、全氟辛烷磺酸(PFOS)、聚全氟辛酸(PFOA)等低聚全氟辛烷磺酸及其衍生物的脱氟产物为碳酸盐、甲酸盐、草酸盐和三氟乙酸盐。这样,全氟辛烷磺酸中的氟就可以作为无机氟化盐回收利用。机理研究揭示了聚四氟乙烯和低聚全氟辛烷磺酸在反应行为和产物成分上的差异。这项研究为 "永恒的化学物质 "PFASs(尤其是聚四氟乙烯)的低温光诱导脱氟以及新型超级光诱导剂的发现打开了一扇窗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


