The biological effects of copper alloying in Zn-based biodegradable arterial implants
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-11-08
DOI:10.1016/j.bioadv.2024.214112
引用次数: 0
Abstract
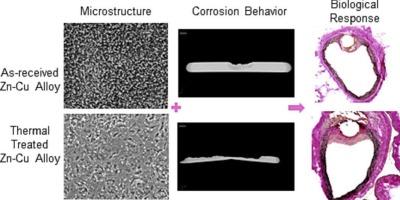
Biodegradable metals based on zinc are being developed to serve as temporary arterial scaffolding. Although the inclusion of copper is becoming more prevalent for grain refinement in zinc alloys, the biological activity of the copper component has not been well investigated. Here, two Zn![]() Cu alloys (0.8 and 1.5 wt% Cu) with and without thermal treatment were investigated for their hemocompatibility and biocompatibility. The microstructure was examined using scanning electron microscopy and X-ray diffraction. Zn-1.5Cu was found to contain nearly double the amount of second phase (CuZn5) precipitates as compared to Zn-0.8Cu. Thermal treatment dissolved a portion of the precipitates into the matrix. Since copper is a well-known catalyst for NO generation, the metals were tested both for their ability to generate NO release and for their thrombogenicity. Cellular responses and in vivo corrosion were characterized by a 6 months in vivo implantation of metal wires into rat arteries. The as-received Zn-1.5Cu displayed the least neointimal growth and smooth muscle cell presence, although inflammation was slightly increased. Thermal treatment was found to worsen the biological response, as determined by an increased neointimal size, increased smooth muscle cell presence and small regions of necrotic tissue. There were no trends in NO release between the alloys and thermal treatments. Corrosion progressed predominately through a pitting mechanism in vivo, which was more pronounced for the thermally treated alloys, with a more uniform corrosion seen for as-received Zn-1.5Cu. Differences in biological response are speculated to be due to changes in microstructure and pitting corrosion behavior.
Cu alloys (0.8 and 1.5 wt% Cu) with and without thermal treatment were investigated for their hemocompatibility and biocompatibility. The microstructure was examined using scanning electron microscopy and X-ray diffraction. Zn-1.5Cu was found to contain nearly double the amount of second phase (CuZn5) precipitates as compared to Zn-0.8Cu. Thermal treatment dissolved a portion of the precipitates into the matrix. Since copper is a well-known catalyst for NO generation, the metals were tested both for their ability to generate NO release and for their thrombogenicity. Cellular responses and in vivo corrosion were characterized by a 6 months in vivo implantation of metal wires into rat arteries. The as-received Zn-1.5Cu displayed the least neointimal growth and smooth muscle cell presence, although inflammation was slightly increased. Thermal treatment was found to worsen the biological response, as determined by an increased neointimal size, increased smooth muscle cell presence and small regions of necrotic tissue. There were no trends in NO release between the alloys and thermal treatments. Corrosion progressed predominately through a pitting mechanism in vivo, which was more pronounced for the thermally treated alloys, with a more uniform corrosion seen for as-received Zn-1.5Cu. Differences in biological response are speculated to be due to changes in microstructure and pitting corrosion behavior.
锌基生物可降解动脉植入物中铜合金的生物效应。
目前正在开发以锌为基础的可生物降解金属,以用作临时动脉支架。虽然在锌合金中加入铜以细化晶粒的做法越来越普遍,但铜成分的生物活性还没有得到很好的研究。在此,我们研究了两种经热处理和未经热处理的锌铜合金(铜含量分别为 0.8 和 1.5 wt%)的血液相容性和生物相容性。使用扫描电子显微镜和 X 射线衍射检查了合金的微观结构。与 Zn-0.8Cu 相比,Zn-1.5Cu 中第二相(CuZn5)沉淀物的含量几乎增加了一倍。热处理将部分沉淀物溶解到基体中。由于铜是众所周知的氮氧化物生成催化剂,因此对这些金属进行了氮氧化物释放能力和血栓形成能力测试。将金属丝植入大鼠动脉 6 个月后,对细胞反应和体内腐蚀进行了鉴定。尽管炎症反应略有增加,但在接受 Zn-1.5Cu 的情况下,新内膜生长和平滑肌细胞的存在最少。热处理会使生物反应恶化,表现为新内膜尺寸增大、平滑肌细胞增多以及坏死组织区域变小。合金和热处理之间的氮氧化物释放量没有变化趋势。体内腐蚀主要是通过点蚀机制进行的,热处理合金的腐蚀更为明显,而原样接收的 Zn-1.5Cu 的腐蚀更为均匀。据推测,生物反应的差异是由于微观结构和点蚀行为的变化造成的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


