Brain drug delivery from the nasal olfactory region is enhanced using lauroylcholine chloride: An estimation using in vivo PET imaging
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
Abstract
Introduction
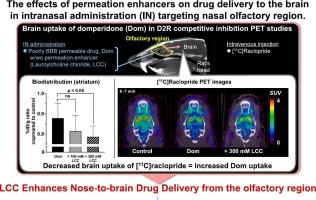
Intranasal (IN) administration, often referred to as nose-to-brain (N2B) drug delivery, is an attractive approach for delivering drugs to the central nervous system. However, the efficacy of this method is limited because of the small size of the nasal olfactory region, which limits the drug dosage. Using permeation enhancers could improve drug delivery from this region to the brain, though their effects are not fully understood. We therefore investigated the effects of co-administration of permeation enhancers on N2B drug delivery of a model drug domperidone, a peripherally acting dopamine D2 receptor (D2R) blocker.
Methods
We conducted in vitro permeability assays to evaluate the effects of sodium lauryl sulfate (SLS), a classical permeation enhancer, and lauroylcholine chloride (LCC) on domperidone permeation in human nasal mucosa-derived cells. We also used the D2R ligand [11C]raclopride to assess the in vivo effects of LCC on domperidone delivery in the rat brain using a positron emission tomography (PET) competition paradigm. In comparative PET experiments, we tested the effects of intravenously administered domperidone without LCC co-administration.
Results
LCC effectively improved nasal mucosal permeation of domperidone in vitro compared to SLS. In rat IN administration experiments, striatal [11C]raclopride uptake was significantly decreased by the addition of LCC to domperidone. On the other hand, intravenously administered domperidone with or without intranasally administered LCC did not decrease [11C]raclopride brain uptake, suggesting a lesser influence of peripheral domperidone on [11C]raclopride brain uptake. PET studies showed that striatal D2R occupancy of domperidone was increased 2.4-fold by co-administration of LCC.
Conclusion
LCC effectively enhances the domperidone delivery from the rat olfactory region to the brain, probably not via a circulating blood. The combination of permeation enhancers and olfactory region-selective drug administration could be effective for N2B drug delivery.
使用氯化月桂酰胆碱可增强从鼻腔嗅区向大脑的药物输送:使用体内 PET 成像进行估算。
导言:鼻内给药通常被称为 "鼻脑给药"(N2B),是向中枢神经系统输送药物的一种极具吸引力的方法。然而,由于鼻腔嗅区较小,限制了药物剂量,因此这种方法的疗效有限。使用渗透促进剂可以改善从这一区域向大脑的药物输送,但其效果尚不完全清楚。因此,我们研究了联合使用渗透促进剂对多潘立酮(一种外周作用的多巴胺 D2 受体(D2R)阻断剂)这种模型药物的 N2B 给药的影响:我们进行了体外渗透性试验,以评估经典渗透促进剂十二烷基硫酸钠(SLS)和氯化月桂酰胆碱(LCC)对多潘立酮在人鼻粘膜衍生细胞中渗透的影响。我们还使用 D2R 配体 [11C]raclopride 通过正电子发射断层扫描(PET)竞争范式评估了 LCC 对大鼠脑内多潘立酮递送的体内影响。在对比 PET 实验中,我们测试了静脉注射多潘立酮而不同时给药 LCC 的效果:结果:与 SLS 相比,LCC 在体外可有效改善多潘立酮的鼻粘膜渗透性。在大鼠 IN 给药实验中,在多潘立酮中添加 LCC 后,纹状体[11C]拉克必利的摄取量明显减少。另一方面,静脉注射多潘立酮与或不静脉注射 LCC 都不会降低大脑对[11C]raclopride 的摄取,这表明外周多潘立酮对大脑摄取[11C]raclopride 的影响较小。正电子发射计算机断层显像研究显示,同时服用 LCC 后,多潘立酮的纹状体 D2R 占有率增加了 2.4 倍:结论:LCC 可有效增强多潘立酮从大鼠嗅区向大脑的输送,而可能不是通过循环血液。渗透促进剂与嗅区选择性给药的结合可有效实现 N2B 给药。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


