Investigation of the susceptibility of clinical infection loads to nitric oxide antibacterial treatment
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
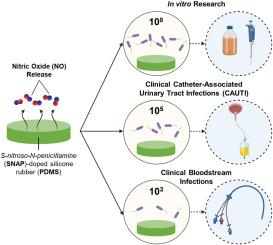
The persistent infection of medical devices by opportunistic pathogens has led to the development of antimicrobial medical device polymers. Nitric oxide (NO) is an endogenous antimicrobial molecule that is released through the degradation of synthetic donor molecules such as S-nitroso-N-acetylpenicillamine (SNAP) embedded into polymer membranes. It is hypothesized that the clinical success of these polymers is enhanced by the physiological release of NO and the consequent prevention of infection. However, such NO-releasing materials have never been evaluated against microbial loads that are commensurate with clinical infection levels. This study aimed to develop a standardized polymer film impregnated with SNAP that consistently releases NO and evaluates its efficacy against bacterial loads that represent clinical infection parameters. Microbial loads of 103, 105, and 108 (colony-forming units) CFU mL−1 were exposed to the NO-releasing polymer, corresponding to bloodstream infections, catheter-associated urinary tract infections, and standard laboratory exposure levels that have been reported in the scientific literature. By 24 h, SNAP films led to >1 log reduction of adhered and viable E. coli at all tested microbial loads compared to control polydimethylsiloxane (PDMS). Further, SNAP films displayed no viable adhered S. aureus at the 103 microbial level for the entire study and showed total planktonic killing by 8 h. NO localization within bacterial cells adhering to the films was evaluated, revealing higher NO uptake and consequent bacterial killing by SNAP samples. This unique study shows that NO-releasing polymers not only kill bacteria adhered to the polymer surface, but localized delivery leads to environmental planktonic bacterial killing that prevents adhesion from occurring. Furthermore, the promising findings of NO-releasing polymers in scientific research indicate their potential for successful application in clinical settings to prevent infections.
临床感染负荷对一氧化氮抗菌治疗敏感性的调查。
机会性病原体对医疗器械的持续感染促使人们开发抗菌医疗器械聚合物。一氧化氮(NO)是一种内源性抗菌气体,可通过嵌入聚合物膜的合成供体分子(如 S-亚硝基-N-乙酰青霉胺(SNAP))的降解释放出来。据推测,NO 的生理性释放可提高这些聚合物的临床疗效,从而预防感染。然而,此类释放 NO 的材料从未针对与临床感染水平相当的微生物负荷进行过评估。本研究旨在开发一种能持续释放 NO 的浸渍 SNAP 标准聚合物薄膜,并评估其对代表临床感染参数的细菌载量的功效。将 103、105 和 108(菌落形成单位)CFU mL-1 的微生物量暴露在释放 NO 的聚合物上,这与科学文献中报道的血流感染、导管相关性尿路感染和标准实验室暴露水平相对应。与对照组聚二甲基硅氧烷(PDMS)相比,在 24 小时内,SNAP 薄膜可使所有测试微生物负荷下的附着和存活大肠杆菌减少 > 1 log。此外,在整个研究过程中,SNAP 薄膜在 103 个微生物水平上没有显示出存活的粘附金黄色葡萄球菌,并在 8 小时内显示出完全的浮游杀菌效果。对粘附在薄膜上的细菌细胞内的 NO 定位情况进行了评估,结果表明 SNAP 样品对 NO 的吸收率更高,从而杀死了更多细菌。这项独特的研究表明,释放 NO 的聚合物不仅能杀死粘附在聚合物表面的细菌,而且局部递送还能杀死环境中的浮游细菌,防止发生粘附。此外,氮氧化物释放聚合物在科学研究中取得的令人鼓舞的成果表明,它们有望成功应用于临床环境,预防感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


