Hypoxia-responsive liposome enhances intracellular delivery of photosensitizer for effective photodynamic therapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Liposomes, especially polyethylene glycol (PEG)-modified long-circulating liposomes, have been approved for market use, due to good biocompatibility, passive tumor targeting, and sustained drug release. PEG-modified long-circulating liposomes address issues such as poor stability and rapid clearance by the reticuloendothelial system. However, they still face challenges like hindering drug uptake by tumor cells and preventing tumor penetration. Inspired by the hypoxic tumor microenvironment, we constructed a hypoxia-responsive liposome (PAO-L) to enhance the intracellular uptake and photodynamic therapy (PDT) effect of chlorin e6 (Ce6). The intelligent hypoxia-cleavable PEG-AZO-OA (PAO) was prepared by coupling PEG and octadecylamine (OA) to hypoxia-sensitive azobenzene-4,4′-dicarboxylic acid (AZO) through amide reaction. The synthesized PAO was further incorporated into Ce6-loaded liposomes to enhance the circulation stability, while promote the tumor penetration and internalization by the responsive shedding of PEG from liposome surface upon reaching the hypoxic tumor tissue. PAO-L mediated PDT significantly inhibited the growth of B16F10 and 4T1 tumors, as well as lung metastasis of 4T1 breast cancer. The excellent therapeutic effect and good tolerability make PAO-L a promising candidate for enhanced PDT.
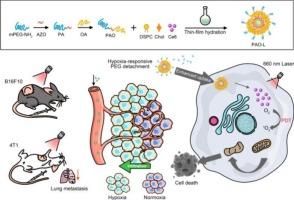
低氧响应脂质体可增强光敏剂的细胞内输送,从而实现有效的光动力疗法。
脂质体,尤其是聚乙二醇(PEG)修饰的长循环脂质体,由于具有良好的生物相容性、被动肿瘤靶向性和药物持续释放性,已被批准上市使用。PEG 改性长循环脂质体解决了稳定性差和被网状内皮系统快速清除等问题。然而,它们仍然面临着阻碍肿瘤细胞吸收药物和阻止肿瘤穿透等挑战。受缺氧肿瘤微环境的启发,我们构建了一种缺氧响应脂质体(PAO-L),以增强细胞内对氯素e6(Ce6)的吸收和光动力疗法(PDT)效果。通过酰胺反应将 PEG 和十八胺(OA)偶联到对缺氧敏感的偶氮苯-4,4'-二羧酸(AZO)上,制备了智能型可清除缺氧的 PEG-AZO-OA(PAO)。合成的 PAO 被进一步加入到 Ce6 脂质体中,以增强其循环稳定性,同时在到达缺氧的肿瘤组织时,PEG 会从脂质体表面响应性脱落,从而促进肿瘤的穿透和内化。PAO-L 介导的光动力疗法显著抑制了 B16F10 和 4 T1 肿瘤的生长以及 4 T1 乳腺癌的肺转移。出色的治疗效果和良好的耐受性使 PAO-L 有望成为增强型 PDT 的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


