Continuous twin-screw melt granulation of drug-loaded electrospun fibers
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-17
DOI:10.1016/j.ejpb.2024.114580
引用次数: 0
Abstract
Electrospinning (ES) is a promising continuous formulation strategy to produce amorphous solid dispersions (ASDs) and thereby improve the dissolution of poorly water-soluble drugs. However, processing the electrospun material into solid dosage forms (e.g. tablets) is challenging due to the poor flow properties. In this research, continuous twin-screw melt granulation was applied to improve the flowability of the fibers and therefore ease the further processing steps. During this work, two ASD compositions were investigated: one containing 60 % poly-vinylpyrrolidone-vinyl acetate 6:4 copolymer and 40 % itraconazole (ITR), and another one containing hydroxypropyl methylcellulose (HPMC) and ITR in the same ratio. Both fiber compositions were granulated with polyethene glycol as the binder material, while the effects of the process parameters were examined. The application of higher granulation temperature and screw configurations with increased shear forces compromised the fibrous structure, induced crystallization of the ASD, and decreased the dissolution. However, the stability of the ITR-HPMC fibers proved to be higher as their granulation at 60 °C led to granules with adequate flow properties and dissolution. Moreover, tablets with fewer excipients were pressed from them, resulting in a 34 % reduction in weight. Consequently, this process can complement ES technology and facilitate its industrial implementation.
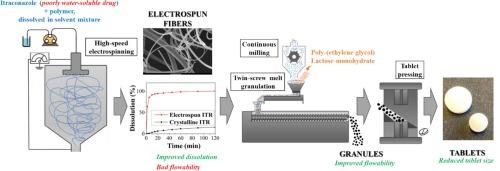
载药电纺纤维的连续双螺杆熔融造粒。
电纺丝(ES)是一种很有前景的连续制剂策略,可用于生产无定形固体分散体(ASD),从而改善水溶性差药物的溶解性。然而,由于电纺材料流动性差,将其加工成固体剂型(如片剂)具有挑战性。在这项研究中,采用了连续双螺杆熔融造粒技术来改善纤维的流动性,从而简化进一步的加工步骤。在这项工作中,研究了两种 ASD 组合物:一种含有 60% 的聚乙烯吡咯烷酮-醋酸乙烯酯 6:4 共聚物和 40% 的伊曲康唑(ITR),另一种含有相同比例的羟丙基甲基纤维素(HPMC)和伊曲康唑(ITR)。两种纤维组合物均以聚乙二醇为粘合剂材料进行造粒,同时考察了工艺参数的影响。较高的造粒温度和剪切力增大的螺杆配置破坏了纤维结构,导致 ASD 结晶,并降低了溶解度。然而,事实证明,ITR-HPMC 纤维的稳定性更高,因为它们在 60 °C 下制粒可获得具有适当流动性和溶解性的颗粒。此外,用它们压制出的片剂辅料更少,重量减轻了 34%。因此,该工艺可作为 ES 技术的补充,并促进其工业化应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


