C1GALT1 high expression enhances the progression of glioblastoma through the EGFR-AKT/ERK cascade
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Core1 β1,3-galactosyltransferase (C1GALT1) is an essential glycotransferase controlling the elongation of GalNAc-type O-glycosylation and its altered expression contributes tumor progression in various cancers. However, the mechanism how C1GALT1 influences gliomas remains unclear. Here,our results from The Cancer Genome Atlas (TCGA) database, The Chinese Glioma Genome Atlas (CGGA) database and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database showed that the expression of C1GALT1 was increased in higher grade gliomas namely glioblastoma compared with low grade gliomas or non-tumor tissues and significantly associated with poor survival. Downregulation of C1GALT1 suppressed cell proliferation, invasion, and migration in glioma cell lines. Consistent with the result in vitro, C1GALT1 knockdown distinctly inhibited the weight and tumor growth in nude mice. Mechanistically, C1GALT1 knockdown decreased the level of terminal galactose O-glycosylation and phosphorylation on epidermal growth factor receptor (EGFR). Moreover, The AKT/ERK phosphorylation was attenuated in C1GALT1 knockdown cells. And C1GALT1 knockdown decreased the expression of cyclinD1, matrix metalloproteinase 9 (MMP9) through the AKT/ERK signaling pathway Furthermore, transcription factor SP1 which the expression was found to be associated the C1GALT1 expression could bind to the promoter of C1GALT1 gene and regulated its expression. In conclusion, our data show that C1GALT1 enhances the progression of glioma by regulated the O-glycosylation and phosphorylation of EGFR and the subsequent downstream AKT/ERK signaling pathway. Therefore, C1GALT1 represents a potential target for the diagnosis and treatment of glioma.
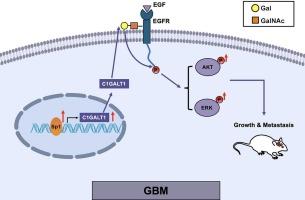
C1GALT1的高表达会通过表皮生长因子受体-AKT/ERK级联反应促进胶质母细胞瘤的进展。
核心1 β1,3-半乳糖基转移酶(C1GALT1)是一种重要的糖基转移酶,控制着GalNAc型O-糖基化的延伸,其表达的改变有助于各种癌症的肿瘤进展。然而,C1GALT1如何影响胶质瘤的机制仍不清楚。在此,我们从癌症基因组图谱(TCGA)数据库、中国胶质瘤基因组图谱(CGGA)数据库和临床肿瘤蛋白质组学分析联盟(CPTAC)数据库中获得的结果显示,与低级别胶质瘤或非肿瘤组织相比,C1GALT1在高级别胶质瘤(即胶质母细胞瘤)中的表达增加,并与不良生存率显著相关。下调 C1GALT1 可抑制胶质瘤细胞系的细胞增殖、侵袭和迁移。与体外实验结果一致,C1GALT1基因敲除明显抑制了裸鼠的体重和肿瘤生长。从机理上讲,C1GALT1基因敲除降低了表皮生长因子受体(EGFR)上的末端半乳糖O-糖基化和磷酸化水平。此外,C1GALT1敲除细胞中的AKT/ERK磷酸化也有所减弱。此外,研究还发现,与C1GALT1表达相关的转录因子SP1能与C1GALT1基因启动子结合并调控其表达。总之,我们的数据表明,C1GALT1通过调控表皮生长因子受体的O-糖基化和磷酸化以及随后的下游AKT/ERK信号通路,促进胶质瘤的进展。因此,C1GALT1是诊断和治疗胶质瘤的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


