Design, synthesis, and biological evaluation of novel highly potent FXR agonists bearing piperidine scaffold
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
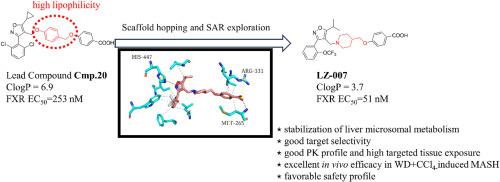
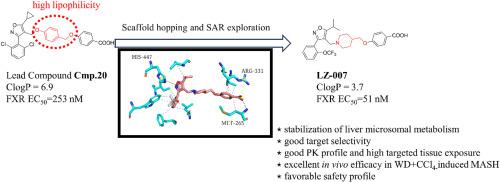
Metabolic dysfunction-associated steatohepatitis (MASH) has become a serious threat to human health, which exhibited an increasing prevalence globally. Recently, the farnesoid X receptor (FXR) has been identified as a promising strategy for the treatment of MASH by regulating multiple pathogenesis. In this study, a new series of FXR agonists bearing piperidine scaffold was designed to reduce the high lipophilicity of the existing FXR agonists. After comprehensive multiparameter optimization, LZ-007 was discovered as a highly potent FXR agonist with suitable stability in liver microsomes of multiple species. LZ-007 exhibited highly oral bioavailability and targeted tissue exposure in the liver and ileum, while the plasma exposure is low, which might minimize the systemic side effects. Moreover, LZ-007 was significantly up-regulated the expressions of FXR and its downstream genes in the liver and ileum. In MASH model, LZ-007 exerted potent anti-MASH effects by regulating the multiple signal pathways related to lipid metabolism, oxidative stress, inflammation and fibrosis. In a 30-day toxicity study, no apparent adverse effects were observed in LZ-007 treated groups, even at the high doses of 250 and 500 mg/kg. With the positive pharmacodynamics and safety profiles, LZ-007 is worthy of further evaluation as a new anti-MASH agent.
带有哌啶支架的新型强效 FXR 激动剂的设计、合成和生物学评价
代谢功能障碍相关性脂肪性肝炎(MASH)已成为严重威胁人类健康的疾病,其发病率在全球呈上升趋势。最近,法尼类固醇 X 受体(FXR)通过调节多种发病机制被认为是治疗 MASH 的一种有前途的策略。本研究设计了一系列带有哌啶支架的新型 FXR 激动剂,以降低现有 FXR 激动剂的高亲脂性。经过全面的多参数优化,LZ-007 被发现是一种高效的 FXR 激动剂,在多个物种的肝脏微粒体中具有合适的稳定性。LZ-007具有很高的口服生物利用度,在肝脏和回肠中的目标组织暴露量较高,而血浆暴露量较低,这可能会将全身副作用降至最低。此外,LZ-007还能显著上调肝脏和回肠中FXR及其下游基因的表达。在MASH模型中,LZ-007通过调节与脂质代谢、氧化应激、炎症和纤维化相关的多种信号通路,发挥了强有力的抗MASH作用。在为期30天的毒性研究中,LZ-007治疗组未观察到明显的不良反应,即使是250毫克/千克和500毫克/千克的高剂量也是如此。LZ-007具有良好的药效学和安全性,值得作为一种新型抗MASH药物进行进一步评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


