Recovery of Li/Co from spent lithium-ion battery through iron-air batteries
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The widespread application of Lithium-ion Batteries (LIBs) has led to a significant increase in the number of spent LIBs. Consequently, the recycling and utilization of spent LIBs have become an inevitable choice for recovering valuable resources and protecting the environment, aligning with the principles of sustainable development. This study reports an iron air battery recycling system that is capable of recovering both Li/Co and energy from spent LiCoO2 cathode materials and the separation reaction is completely spontaneous. Meanwhile, lithium and cobalt were successfully recovered from the powdered leach solution of spent LiCoO2 cathode material, and it was preliminarily demonstrated that spent iron could be used as a sacrificial anode for the system. Several key parameters affecting recovery are optimized, including anode solution pH, discharge current density, and concentration of the solution to be recovered. Under the conditions of anode solution pH of 1.8, discharge current density of 0.1 mA cm−2, and concentration of recovery solution of 0.15 mol/L, the system can be operated stably for more than 24 h, and the recoveries of Li+ and Co2+ can be up to 1.49 mg h−1 and 18.75 mg h−1 respectively, and the output energy can reach 75.15 kJ/mol. This research not only provides a sustainable and cost-effective method of disposing of used lithium-ion batteries, but also offers up new possibilities for the disposal of scrap iron.
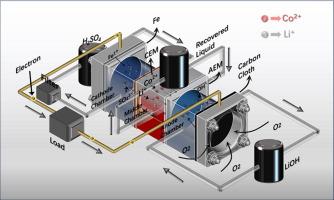
通过铁-空气电池从废锂离子电池中回收锂/钴
锂离子电池(LIB)的广泛应用导致废锂离子电池的数量大幅增加。因此,废锂电池的回收和利用已成为回收宝贵资源和保护环境的必然选择,符合可持续发展的原则。本研究报道了一种铁空气电池回收系统,该系统能够从废旧钴酸锂正极材料中同时回收锂/钴和能量,且分离反应完全自发。同时,从废钴酸锂正极材料的粉末浸出液中成功回收了锂和钴,并初步证明废铁可用作该系统的牺牲阳极。对影响回收的几个关键参数进行了优化,包括阳极溶液的 pH 值、放电电流密度和待回收溶液的浓度。在阳极溶液 pH 值为 1.8、放电电流密度为 0.1 mA cm-2、回收溶液浓度为 0.15 mol/L 的条件下,系统可稳定运行 24 小时以上,Li+ 和 Co2+ 的回收率分别达到 1.49 mg h-1 和 18.75 mg h-1,输出能量达到 75.15 kJ/mol。这项研究不仅为处理废旧锂离子电池提供了一种可持续且经济有效的方法,也为废铁的处理提供了新的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


