MCL1 Inhibitor Augmented the Anti-Glioma Efficacy of Paclitaxel Utilizing a Multifunctional Cascade Nanodrug System
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
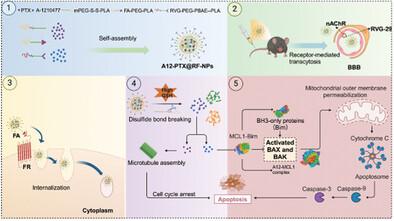
Despite the importance of chemotherapy as a treatment option for glioma, its efficacy is often compromised by the formidable blood-brain barrier (BBB) and drug resistance. To address these challenges, a novel cascade nanodrug system called A12-PTX@RF-NPs is designed with aims to penetrate the BBB and precisely target glioma. In this nanosystem, the RVG-29 peptide facilitates the BBB penetration while Folic Acid (FA) targets glioma cells through binding to Folate Receptors (FR), followed by receptor-mediated endocytosis subsequently. The incorporation of disulfide bond modifications enables responsive release within the reductive environment of glioma, ensuring successful delivery of chemotherapy drugs. Significantly, a co-treatment approach involving the combination of A12 and PTX is implemented. In vitro and in vivo investigations have provided evidence that this amalgamation effectively induces apoptosis in tumor cells and inhibits their proliferation, thus synergistically eliminating both typical and drug-resistant glioma cells. These findings suggest that the nanodrug system presents a promising therapeutic strategy for glioma treatment, surpassing the limitations of conventional chemotherapy. Specifically, A12-PTX@RF-NPs constructed in this research have demonstrated remarkable targeting capabilities and therapeutic effects in cellular as well as animal models, thereby proposing an innovative strategy for glioma treatment.
利用多功能级联纳米药物系统,MCL1 抑制剂增强了紫杉醇的抗胶质瘤疗效
尽管化疗是治疗脑胶质瘤的重要手段,但其疗效往往会因难以逾越的血脑屏障(BBB)和耐药性而大打折扣。为了应对这些挑战,我们设计了一种名为 A12-PTX@RF-NPs 的新型级联纳米药物系统,旨在穿透 BBB 并精确靶向胶质瘤。在这一纳米系统中,RVG-29 多肽有助于穿透 BBB,而叶酸(FA)则通过与叶酸受体(FR)结合,随后通过受体介导的内吞作用靶向胶质瘤细胞。二硫键修饰的加入可在胶质瘤的还原环境中实现反应性释放,从而确保化疗药物的成功输送。值得注意的是,A12 和 PTX 的联合治疗方法已经实现。体外和体内研究证明,这种组合能有效诱导肿瘤细胞凋亡并抑制其增殖,从而协同消灭典型的和耐药的胶质瘤细胞。这些发现表明,纳米药物系统超越了传统化疗的局限性,为胶质瘤治疗提供了一种前景广阔的治疗策略。具体而言,本研究构建的 A12-PTX@RF-NPs 在细胞和动物模型中都表现出了显著的靶向能力和治疗效果,从而为胶质瘤治疗提出了一种创新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


