Molecular engineering, supporting electrolyte, and membrane selections for enhanced cycling stability of non-aqueous organic redox flow batteries: A review
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Redox flow batteries (RFBs) have attracted researchers due to their decoupled nature of energy and power modulations, suitability for large-scale stationary energy storage, and integration of renewable intermittent energy sources such as solar and wind power. Water’s narrow electrochemical stability window limits the energy density of aqueous redox flow batteries. Thus, a shift to non-aqueous organic redox flow batteries (NAORFBs) is necessary to achieve high energy density while benefiting from organic solvents’ expansive electrochemical stability windows. Nonetheless, the degradation and crossover of organic electroactive materials cause rapid capacity loss in NAORFBs. To improve the cycling stability of NAORFBs, molecular engineering is required to enhance the stability of redox-active species, particularly charged species, and the solubility of redox-active species. An appropriate ion-selective membrane that mitigates crossover by selectively allowing the passage of ions of supporting salts needs to be developed. This review discusses molecular design strategies that may improve radical ion stability, increase the solubility of redox-active species, and reduce redox-active species crossover and the selection of appropriate supporting electrolytes and separators/membranes for the overall enhancement of the cycle life and performance.
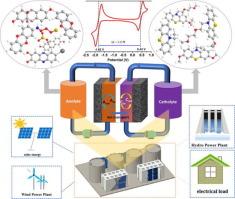
分子工程、支撑电解质和隔膜选择用于增强非水有机氧化还原液流电池的循环稳定性:综述
氧化还原液流电池(RFB)因其能量和功率调节的解耦性、适合大规模固定储能以及可再生间歇性能源(如太阳能和风能)的整合而吸引了研究人员。水的电化学稳定性窗口较窄,限制了水氧化还原液流电池的能量密度。因此,有必要转向非水性有机氧化还原液流电池(NAORFB),以实现高能量密度,同时受益于有机溶剂宽广的电化学稳定性窗口。然而,有机电活性材料的降解和交叉会导致 NAORFB 的容量迅速下降。为了提高 NAORFB 的循环稳定性,需要通过分子工程来增强氧化还原活性物种(尤其是带电物种)的稳定性以及氧化还原活性物种的溶解度。需要开发一种适当的离子选择性膜,通过选择性地允许支持盐类的离子通过来减轻交叉。本综述讨论了可提高自由基离子稳定性、增加氧化还原活性物种溶解度、减少氧化还原活性物种交叉的分子设计策略,以及选择适当的支持电解质和分离器/膜以全面提高循环寿命和性能的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


