Spleen-Targeted mRNA Nanoparticles for Modulating B Cell Hyperactivation in Rheumatoid Arthritis Therapy
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Messenger RNA (mRNA)-based therapies have emerged as a revolutionary strategy for treating various diseases. In autoimmune diseases like rheumatoid arthritis (RA), targeted mRNA delivery provides a potential intervention to modulate immune responses. However, achieving specific and efficient in vivo modulation of immune regulators, such as the inhibitory Fc gamma receptor, FcγRIIB, on B cells remains challenging. In this study, lipid polymer nanoparticles (LPNs) formulated with AMB-POC18 lipidoid and poly(ethylene glycol)-block-polylactide (PEG-PLA) are engineered to deliver FcγRIIB mRNA (mFcγRIIB) specifically to splenic B cells for RA treatment. Protein corona analysis indicated that selective adsorption of complement C3 on the LPNs' surface facilitated their targeted delivery to the spleen, enhancing transfection efficiency in B cells following intravenous administration. In a collagen-induced arthritis mouse model, mFcγRIIB/LPNs effectively upregulated FcγRIIB expression in splenic B cells, significantly reducing autoimmune responses and alleviating RA symptoms. Further mechanistic studies elucidated that increased FcγRIIB expression suppressed B cell activation via the FcγRIIB/Lyn/SHP-1 signaling pathway. This work underscored the potential of the spleen-targeted mRNA delivery system for RA therapy, providing a precise and targeted approach to modulate B cell activity and mitigate autoimmune diseases.
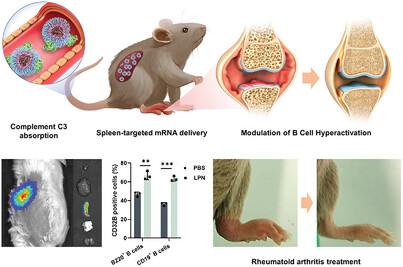
用于调节类风湿性关节炎治疗中 B 细胞过度激活的脾靶向 mRNA 纳米粒子
基于信使核糖核酸(mRNA)的疗法已成为治疗各种疾病的革命性策略。在类风湿性关节炎(RA)等自身免疫性疾病中,靶向 mRNA 递送为调节免疫反应提供了潜在的干预手段。然而,在体内实现对免疫调节因子(如 B 细胞上的抑制性 Fc γ 受体 FcγRIIB)的特异性和高效调节仍具有挑战性。在这项研究中,用 AMB-POC18 类脂质和聚乙二醇-阻聚-聚乳酸(PEG-PLA)配制的脂质聚合物纳米颗粒(LPNs)可将 FcγRIIB mRNA(mFcγRIIB)特异性地输送到脾脏 B 细胞,用于治疗 RA。蛋白电晕分析表明,补体C3在LPN表面的选择性吸附促进了它们向脾脏的定向输送,提高了静脉注射后B细胞的转染效率。在胶原蛋白诱导的关节炎小鼠模型中,mFcγRIIB/LPNs 能有效上调脾脏 B 细胞中 FcγRIIB 的表达,从而显著降低自身免疫反应并减轻 RA 症状。进一步的机理研究阐明,FcγRIIB表达的增加通过FcγRIIB/Lyn/SHP-1信号通路抑制了B细胞的活化。这项研究强调了脾脏靶向 mRNA 运送系统治疗 RA 的潜力,为调节 B 细胞活性和缓解自身免疫性疾病提供了一种精确的靶向方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


