Modeling of the Adsorption of Tigecycline from Water on CoFe2O4-Graphene Nanocomposites
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A CoFe2O4(11.04%)-graphene (5.45%) nanocomposite was synthesized and characterized by spectroscopic techniques. This nanocomposite was used to eliminate tigecycline antibiotics from the water. The adsorbent showed 160.0 mg/g adsorption capacity of tigecycline antibiotic at 175 mg/L tigecycline, 0.75 g/L dose, 100 min of contact time, and a temperature of 25 °C. One-, two-, and three-parameter models were applied, i.e., Henry, Langmuir, Freundlich, D–R, Temkin, Flory–Huggins, Halsey, Jovanovich, Redlich–Peterson, and Sips models. According to statistical data, Langmuir and Sips models were the best fitted. The adsorption was spontaneous thermodynamically following pseudo-second-order kinetics. The adsorption occurred via a combination of intraparticle diffusion and external mass transfer mechanisms. The supramolecular mechanism showed the adsorption of the tigecycline antibiotic via coordination and π–π stacking bonds. The characterization results showed that the average nanoparticle size obtained was 91.45 nm. The removal efficiency of the adsorbent reduced up to the fifth cycle and later became constant at 50%. Hence, CoFe2O4-graphene nanocomposites propose a highly effective and recyclable solution for water treatment through adsorption, and hence, this method may be used to remove tigecycline antibiotics from water bodies.
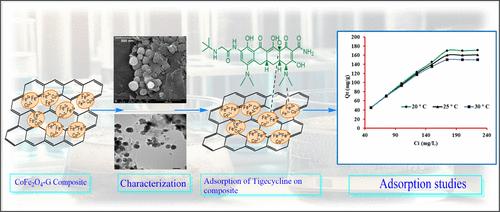
钴铁氧体-石墨烯纳米复合材料对水中替加环素的吸附模拟
研究人员合成了一种 CoFe2O4(11.04%)-石墨烯(5.45%)纳米复合材料,并利用光谱技术对其进行了表征。该纳米复合材料用于去除水中的替加环素抗生素。在虎头环素含量为 175 毫克/升、剂量为 0.75 克/升、接触时间为 100 分钟、温度为 25 ℃ 的条件下,该吸附剂对虎头环素抗生素的吸附量为 160.0 毫克/克。应用了单参数、双参数和三参数模型,即 Henry 模型、Langmuir 模型、Freundlich 模型、D-R 模型、Temkin 模型、Flory-Huggins 模型、Halsey 模型、Jovanovich 模型、Redlich-Peterson 模型和 Sips 模型。根据统计数据,Langmuir 和 Sips 模型的拟合效果最好。吸附是自发的,热力学上遵循伪二阶动力学。吸附是通过颗粒内扩散和外部传质相结合的机制进行的。超分子机理表明,替加环素抗生素是通过配位键和π-π堆积键吸附的。表征结果表明,纳米颗粒的平均粒径为 91.45 nm。吸附剂的去除率在第五个循环之前有所降低,之后稳定在 50%。因此,CoFe2O4-石墨烯纳米复合材料为通过吸附进行水处理提供了一种高效且可回收的解决方案,因此这种方法可用于去除水体中的替加环素抗生素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


