A yeast-based oral therapeutic delivers immune checkpoint inhibitors to reduce intestinal tumor burden
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Engineered probiotics are an emerging platform for in situ delivery of therapeutics to the gut. Herein, we developed an orally administered, yeast-based therapeutic delivery system to deliver next-generation immune checkpoint inhibitor (ICI) proteins directly to gastrointestinal tumors. We engineered Saccharomyces cerevisiae var. boulardii (Sb), a probiotic yeast with high genetic tractability and innate anticancer activity, to secrete “miniature” antibody variants that target programmed death ligand 1 (Sb_haPD-1). When tested in an ICI-refractory colorectal cancer (CRC) mouse model, Sb_haPD-1 significantly reduced intestinal tumor burden and resulted in significant shifts to the immune cell profile and microbiome composition. This oral therapeutic platform is modular and highly customizable, opening new avenues of targeted drug delivery that can be applied to treat a myriad of gastrointestinal malignancies.
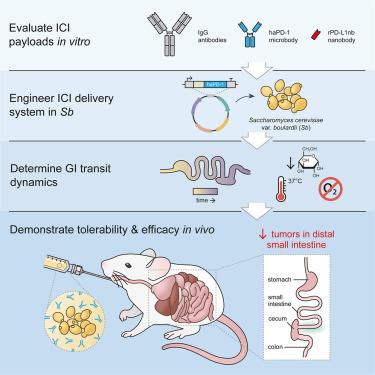

基于酵母的口服疗法可提供免疫检查点抑制剂,减轻肠道肿瘤负担
工程益生菌是向肠道原位递送治疗药物的新兴平台。在此,我们开发了一种基于酵母的口服给药治疗递送系统,可直接向胃肠道肿瘤递送下一代免疫检查点抑制剂(ICI)蛋白。布拉氏酵母(Sb)是一种具有高度遗传可操作性和先天抗癌活性的益生酵母,我们将其改造成能分泌靶向程序性死亡配体1(Sb_haPD-1)的 "微型 "抗体变体。在 ICI 难治性结直肠癌(CRC)小鼠模型中进行测试时,Sb_haPD-1 显著降低了肠道肿瘤负担,并使免疫细胞谱和微生物组组成发生了显著变化。这种口服治疗平台是模块化的,可高度定制,开辟了靶向给药的新途径,可用于治疗各种胃肠道恶性肿瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


