Controlling the Selectivity of Reaction Products by Transmetalation on a Ag(111) Substrate
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
On-surface synthesis has shown great promise in the precise bottom-up preparation of molecular nanostructures. Apart from the direct C–C coupling reaction pathway, an alternative strategy is to exploit the metal–organic interactions provided by integrated metals for preassembly, which exhibit high reversibility and can anchor specific conformations of molecular precursors, thus allowing the precise construction of nanostructures with improved reaction selectivity. Previous studies have mainly been devoted to the construction of target reaction products through the incorporation of metal atoms, ranging from intrinsic to extrinsic atoms on metal substrates and, more recently, to their cooperative effects. However, the formation of different covalent nanostructures by competitive interactions between intrinsic and extrinsic adatoms remains elusive. Herein, we controlled the selectivity of covalent reaction products from isomerically specific trans-chains to cis-rings, resulting from the transmetalation of intrinsic Ag adatoms to extrinsic Na atoms on a Ag(111) substrate. Our results exhibit the competitive interactions between intrinsic and extrinsic metal atoms in real space and demonstrate their influence on the selectivity of reaction products, which should broaden the regulatory strategies for on-surface synthesis that shed light on the controllable and selective synthesis of target covalent nanostructures.
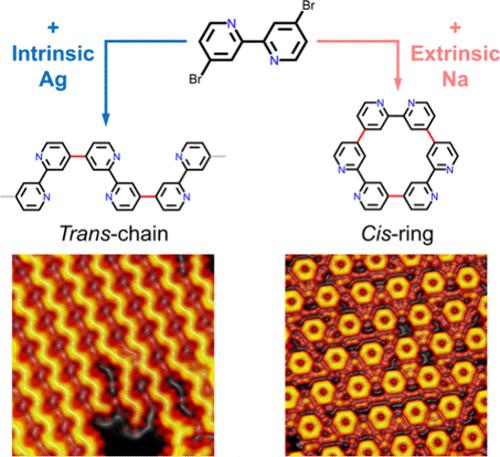
在 Ag(111) 基质上通过跨金属化控制反应产物的选择性
表面合成技术在自下而上精确制备分子纳米结构方面前景广阔。除了直接的 C-C 偶联反应途径外,另一种策略是利用集成金属提供的金属-有机相互作用进行预组装,这种相互作用表现出高度的可逆性,可以锚定分子前体的特定构象,从而可以精确地构建具有更高的反应选择性的纳米结构。以往的研究主要致力于通过在金属基底上掺入金属原子(从固有原子到外在原子)来构建目标反应产物,最近的研究则涉及金属原子的协同效应。然而,通过本征原子和外征原子之间的竞争性相互作用形成不同的共价纳米结构仍是一个未知数。在此,我们控制了共价反应产物从同分异构反式链到顺式环的选择性,这是由 Ag(111) 基质上的固有银原子与外在 Na 原子的反金属化作用产生的。我们的研究结果展示了本征金属原子和外征金属原子在真实空间中的竞争性相互作用,并证明了它们对反应产物选择性的影响,这将拓宽表面合成的调控策略,为目标共价纳米结构的可控性和选择性合成提供启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


