A Near-Infrared II Photo-Triggered Multifunctional Plasmonic Hyperthermia Immunomodulator for SERS-Guided Combination Cancer Immunotherapy
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Immunotherapy represents a promising therapeutic strategy for cancer treatment, but its clinical applications are currently hindered by insufficient therapeutic potency, nonspecific delivery, and adverse side effects. Herein, a novel near-infrared II (NIR-II) photo-triggered plasmonic hyperthermia immunomodulator (RP@IR-pcNS@HA nanoparticles (NPs)) for anticancer treatment of both primary and distant cancers is reported. This immunomodulator comprises an IR-1061 dye-encoded NIR-II porous cubic AuAg nanoshell (pcNS) loaded with a Toll-like receptor 7 agonist – R837 in phase change materials (PCMs), further modified with hyaluronic acid (HA). In response to NIR-II photoirradiation, the RP@IR-pcNS@HA NPs controllably deliver and release R837 to tumor sites, subsequently perform plasmonic hyperthermia therapy for direct ablation of primary tumors, and elicit robust anticancer immune responses. It is demonstrated that upon NIR-II irradiation, such a plasmonic hyperthermia immunomodulator combined with anti-programmed death 1 antibody (αPD-1) completely eradicates both primary and distant cancers. In addition, this combination treatment successfully elicits robust immune memory responses for effective suppression of recurrence and distant metastasis of cancer. With the excellent NIR-II surface-enhanced Raman scattering (SERS) detection ability, the RP@IR-pcNS@HA NPs combined with αPD-1 represent an efficient way to develop high-performance theranostic agents for SERS-guided combination cancer photoimmunotherapy.
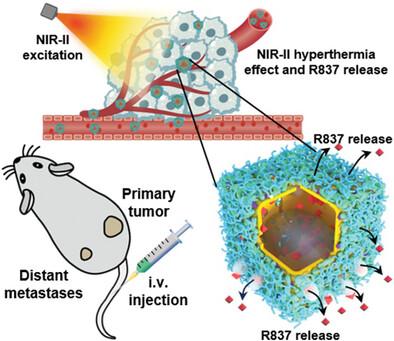
用于 SERS 引导的联合癌症免疫疗法的近红外 II 光触发多功能质子高热免疫调节剂
免疫疗法是一种很有前景的癌症治疗策略,但其临床应用目前受到治疗效力不足、非特异性递送和不良副作用的阻碍。本文报告了一种新型的近红外II(NIR-II)光触发质子高热免疫调节剂(RP@IR-pcNS@HA纳米颗粒(NPs)),用于原发性和远处癌症的抗癌治疗。这种免疫调节剂由 IR-1061 染料编码的近红外-II 多孔立方金银纳米壳(pcNS)组成,在相变材料(PCMs)中装载了 Toll 样受体 7 激动剂 R837,并进一步用透明质酸(HA)修饰。在近红外-II 光照射下,RP@IR-pcNS@HA NPs 可控地向肿瘤部位输送和释放 R837,随后进行等离子体热疗以直接消融原发性肿瘤,并激发强有力的抗癌免疫反应。实验证明,在近红外-II辐照下,这种质子高热免疫调节剂与抗程序性死亡 1 抗体(αPD-1)相结合,可彻底消除原发性和远处的癌症。此外,这种联合疗法还能成功激发强大的免疫记忆反应,从而有效抑制癌症的复发和远处转移。RP@IR-pcNS@HA NPs具有出色的近红外-II表面增强拉曼散射(SERS)检测能力,与αPD-1的结合是开发SERS引导的联合癌症光免疫疗法高性能治疗剂的有效途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


