Understanding the working principle of sustainable catalytic materials for selective hydrogenation of carbonyls bond in α, β-unsaturated aldehydes
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
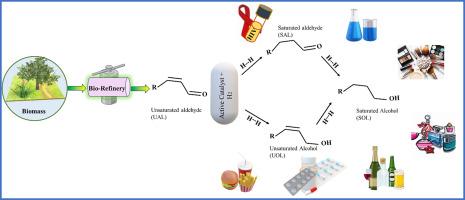
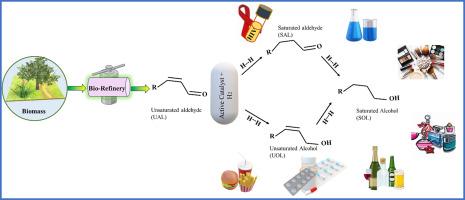
The superior hydrogenation of carbonyl (C=O) bonds in α, β-unsaturated aldehydes (UAL) has attracted considerable attention from economic and industrial perspectives. Several efforts have been made because hydrogenation of the olefin (C![]() C) bond is kinetically and thermodynamically preferred over C
C) bond is kinetically and thermodynamically preferred over C![]() O bond hydrogenation. Hence, to achieve superior hydrogenation of the targeted C
O bond hydrogenation. Hence, to achieve superior hydrogenation of the targeted C![]() O bond, highly active and durable catalysts are required. Herein, functional hydrogenation catalytic materials and their working principles are thoroughly discussed and apprehended. The active role of noble, non-noble mono/bi-metal catalysts and support materials along with the key factors arising from the structure of catalysts that promotes the C
O bond, highly active and durable catalysts are required. Herein, functional hydrogenation catalytic materials and their working principles are thoroughly discussed and apprehended. The active role of noble, non-noble mono/bi-metal catalysts and support materials along with the key factors arising from the structure of catalysts that promotes the C![]() O bond selectivity were thoroughly disclosed. Essential techniques and strategies, such as tuning the surface electronic properties and generating electro-nucleophilic sites via synergistic effects, geometric effects, and applying a confinement or steric effect for improved C
O bond selectivity were thoroughly disclosed. Essential techniques and strategies, such as tuning the surface electronic properties and generating electro-nucleophilic sites via synergistic effects, geometric effects, and applying a confinement or steric effect for improved C![]() O bond hydrogenation, are briefly apprehended. The aggregate analysis suggested two crucial approaches for the engineering of a vastly selective and stable hydrogenation catalytic material: (1) tuning the electronic number of an active noble metal and (2) stabilizing the active noble metal by selecting distinctive support materials, especially metal-organic frameworks (MOFs) owing to its special physiochemical features, to construct robust metal-support interactions. In the end, various crucial key factors and additional active sites that are also encountered to attain the desired selectivity of the C
O bond hydrogenation, are briefly apprehended. The aggregate analysis suggested two crucial approaches for the engineering of a vastly selective and stable hydrogenation catalytic material: (1) tuning the electronic number of an active noble metal and (2) stabilizing the active noble metal by selecting distinctive support materials, especially metal-organic frameworks (MOFs) owing to its special physiochemical features, to construct robust metal-support interactions. In the end, various crucial key factors and additional active sites that are also encountered to attain the desired selectivity of the C![]() O bond are concisely reviewed. Regardless of the numerous successes, significant development is still essential to expand our understanding of the preferential hydrogenation of challenging C
O bond are concisely reviewed. Regardless of the numerous successes, significant development is still essential to expand our understanding of the preferential hydrogenation of challenging C![]() O bonds in UAL.
O bonds in UAL.
了解用于选择性氢化α、β-不饱和醛中羰基键的可持续催化材料的工作原理
从经济和工业的角度来看,α、β-不饱和醛(UAL)中羰基(C=O)键的氢化性能优越,引起了广泛关注。由于烯烃(CC)键的氢化在动力学和热力学上都优于 CO 键的氢化,因此人们做出了许多努力。因此,要实现目标 CO 键的良好氢化,需要高活性和耐久性催化剂。本文对功能性氢化催化材料及其工作原理进行了深入探讨和理解。深入揭示了惰性、非惰性单/双金属催化剂和支撑材料的活性作用,以及催化剂结构中促进 CO 键选择性的关键因素。此外,还简要介绍了一些重要的技术和策略,如通过协同效应、几何效应和应用限制或立体效应来调整表面电子特性和产生亲电核位点,以改善 CO 键氢化。综合分析表明了设计具有高度选择性和稳定性的氢化催化材料的两个关键方法:(1) 调节活性贵金属的电子数;(2) 通过选择独特的支撑材料,尤其是具有特殊理化特性的金属有机框架(MOFs)来稳定活性贵金属,从而构建稳健的金属-支撑相互作用。最后,还简明扼要地回顾了为获得理想的一氧化碳键选择性而遇到的各种关键因素和额外的活性位点。尽管取得了众多成功,但要加深我们对 UAL 中具有挑战性的 CO 键优先氢化的理解,仍有必要取得重大进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


