Electrochemical CO2 Reduction by Heterogeneous Catalysts of 2D Metal-Organic Frameworks Comprising Metal-Coordinated Porphyrins
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
In this study, we employed first-principles computations to design two-dimensional (2D) metal organic framework (MOF) electrocatalysts with metal-porphyrin nanosheets (TM-TCPP-MOF), for CO2 electroreduction. We investigated 3d-transition metal doping on the porphyrin units to screen for efficient catalysts. Structural stability, including thermodynamic and electrochemical stability, was evaluated through binding energy, cohesive energy, formation energy, and dissolution potential analyses. Our results show that TM-TCPP-MOF catalysts are both thermodynamically and electrochemically stable, and the predicted Gibbs free energy profiles indicate HCOOH and CO as the most likely products. The stability of *COOH on Fe-, Co-, Ni-, and Cu-TCPP-MOF leads to CO formation, while *OCHO stabilization on Sc-, Ti-, V-, Cr-, and Mn-TCPP-MOF favors HCOOH production. The competitive hydrogen evolution reaction (HER) was evaluated to study the selectivity. Cr-TCPP-MOF and Co-TCPP-MOF exhibit superior selectivity and activity for CO2 electroreduction.
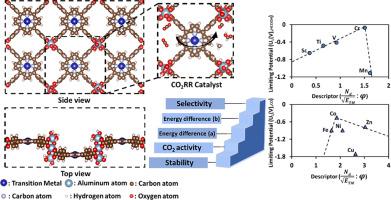
由金属配位卟啉组成的二维金属有机框架异质催化剂的二氧化碳电化学还原作用
在本研究中,我们利用第一性原理计算设计了具有金属卟啉纳米片(TM-TCPP-MOF)的二维(2D)金属有机框架(MOF)电催化剂,用于二氧化碳的电还原。我们研究了卟啉单元上的 3d 过渡金属掺杂,以筛选高效催化剂。我们通过结合能、内聚能、形成能和溶解势分析评估了结构稳定性,包括热力学和电化学稳定性。结果表明,TM-TCPP-MOF 催化剂在热力学和电化学方面都很稳定,预测的吉布斯自由能曲线表明 HCOOH 和 CO 最有可能成为产物。*COOH在Fe-、Co-、Ni-和Cu-TCPP-MOF上的稳定性导致了CO的生成,而*OCHO在Sc-、Ti-、V-、Cr-和Mn-TCPP-MOF上的稳定性则有利于HCOOH的生成。为了研究选择性,对竞争性氢进化反应(HER)进行了评估。Cr-TCPP-MOF 和 Co-TCPP-MOF 在 CO2 电还原反应中表现出更高的选择性和活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


