Preparation and Carbon Nanotube Modification of High Voltage LiNi0.5Mn1.5O4 Cathode Materials
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
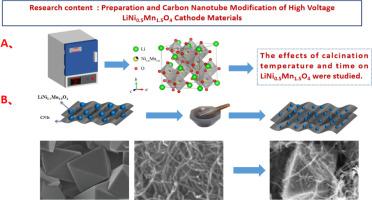
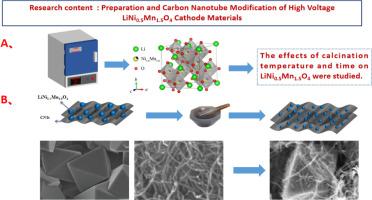
In this study, the nickel-manganese binary cathode material was synthesized using a high-temperature solid-state roasting method. The effects of roasting temperature and roasting time on the electrochemical performance of the cathode material was investigated. The research demonstrated that under conditions of 850 °C for 12 h, the synthesized LNMO exhibited an Fd-3 m space group structure characterized. The initial discharge-specific capacity reached 135 mAh·g⁻¹ at a rate of 0.2C, while the capacity retention rate was 91.8 % after 100 cycles. Following the modification with carbon nanotubes, exhibited an initial discharge capacity of 140 mAh·g⁻¹ at a rate of 0.2 C, maintaining a capacity retention rate of 92.9 % after 100 cycles. Carbon nanotube modification was implemented to address the poor conductivity and unstable electrochemical performance of LNMO when utilizing acetylene black as a conductive agent. This finding suggests that the unique three-dimensional conductive network formed by carbon nanotube offers superior modification effects compared to acetylene black, while the reduced quantity of conductive agent used also contributes to cost savings.
高压 LiNi0.5Mn1.5O4 阴极材料的制备与碳纳米管改性
本研究采用高温固态焙烧法合成了镍锰二元阴极材料。研究了焙烧温度和焙烧时间对阴极材料电化学性能的影响。研究表明,在 850°C 条件下焙烧 12 小时,合成的 LNMO 表现出 Fd-3m 空间群结构特征。在 0.2C 放电速率下,初始放电特定容量达到 135 mAh-g-¹,100 次循环后容量保持率为 91.8%。使用碳纳米管改性后,在 0.2C 放电速率下的初始放电容量为 140 mAh-g-¹,100 次循环后的容量保持率为 92.9%。碳纳米管改性是为了解决 LNMO 在使用乙炔黑作为导电剂时导电性差和电化学性能不稳定的问题。这一发现表明,与乙炔黑相比,碳纳米管形成的独特三维导电网络具有更优越的改性效果,同时导电剂用量的减少也有助于节约成本。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


