Preparation of β-AlF3 from fluorine-containing wastes: Leaching with fluorosilicic acid and crystallization
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Numerous fluorine-containing hazardous solid wastes from the electrolytic aluminum process pose a serious threat to the ecosystem and human health. Currently, in the study of leaching on these wastes, Al and F are usually recovered by adjusting the pH of the leaching solution to precipitate aluminium hydroxyfluoride hydrate, which can be used to produce aluminum fluoride by roasting. However, aluminium hydroxyfluoride hydrate is often mingled with cryolite and other impurities when precipitating, which ultimately affects the purity of obtained aluminum fluoride by calcination. Interestingly, herein, the aluminium hydroxyfluoride hydrate residue was digested by fluorosilicic acid to effectively remove impurities and obtain a pure aluminum fluoride solution, from which the β-AlF3 product was produced by crystallization at a high-temperature. The results show that under the conditions of a temperature of 60 °C, time of 35 min, initial fluorine-aluminum molar ratio of 3:1 and initial concentration of fluorosilicic acid of 18 %, the gross yield of fluorine is 86.2 %, and the recovery of silicon in the form of SiO2 is 95.2 %. During crystallization, the product changes from AlF3·3H2O to β-AlF3 with the increase of temperature. Under the conditions of a crystallization temperature of 150 °C, an initial concentration of aluminum fluoride of 191.10 g/L and a stirring speed of 200 rpm, β-AlF3 of an average particle size of 43.22 μm was obtained by adding 5 % seed. The contents of Al and F in β-AlF3 products are 32.57 % and 61.49 % respectively, which meet the requirements of GB/T 4292–2017 (AF-0) about National Standards of China. According to DFT calculation, the β-AlF3 tends to adsorb two or three water molecules in its cavity structure, which explains why the crystallized β-AlF3 contains water of 5.10 % at 180 °C.
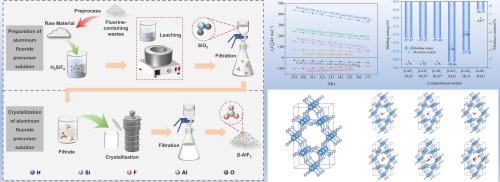
从含氟废物中制备β-AlF3:用氟硅酸浸出和结晶
电解铝工艺产生的大量含氟危险固体废物对生态系统和人类健康构成严重威胁。目前,在对这些废物进行浸出研究时,通常是通过调节浸出液的 pH 值沉淀出水合羟氟化铝来回收铝和氟,然后通过焙烧生产氟化铝。然而,氢氟化铝水合物在沉淀时往往会混入冰晶石和其他杂质,最终影响煅烧得到的氟化铝的纯度。有趣的是,本文用氟硅酸消化羟基氟化铝水合物残渣,有效地除去了杂质,得到了纯净的氟化铝溶液,并从该溶液中通过高温结晶制得了β-AlF3 产物。结果表明,在温度为 60 ℃、时间为 35 分钟、初始氟铝摩尔比为 3:1 和氟硅酸初始浓度为 18% 的条件下,氟的总产率为 86.2%,以 SiO2 形式存在的硅的回收率为 95.2%。在结晶过程中,随着温度的升高,产物由 AlF3-3H2O 变为 β-AlF3。在结晶温度为 150 °C、氟化铝初始浓度为 191.10 g/L、搅拌速度为 200 rpm 的条件下,加入 5 % 的种子可得到平均粒径为 43.22 μm 的 β-AlF3。β-AlF3产品中Al和F的含量分别为32.57%和61.49%,符合中国国家标准GB/T 4292-2017(AF-0)的要求。根据 DFT 计算,β-AlF3 在其空腔结构中倾向于吸附两个或三个水分子,这解释了为什么在 180 °C 时结晶的 β-AlF3 含水量为 5.10%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


