Switching peroxymonosulfate activation pathway from free radical to surface-bound radical over MnFe2O4 for enhanced degradation of ofloxacin: Key role of size effect and surface hydroxyl group
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The modulation of peroxymonosulfate (PMS) activation pathway to achieve effective degradation of pollutants is significant, but still challenging. Herein, a series of hydroxyl- and size-controlled MnFe2O4 catalysts were synthesized through an alkaline microenvironment regulation strategy. The variable-sized MnFe2O4 (submicron, nanoscale and microscale) exhibited size-dependent catalytic behavior, while the changes in surface hydroxyl content altered the activation pathway from dissolved radicals to surface-bound radicals. The quenching experiments, electron spin resonance spectroscopy, electrochemical studies, in-situ Raman spectra and density functional theory calculations were conducted to reveal the evolution of reactive oxygen species. Due to strong binding energy, PMS was stabilized by the rich surface hydroxyl to form surface complexed MnFe2O4-HOOSO3− and simultaneously activated by the active bimetallic components, resulting in oriented-production of surface-bonded radicals. Benefiting from smaller particle size and rich hydroxyl groups, the optimal nano-MnFe2O4-OH/PMS system could massively generate surface-bound SO4•−, which achieved a highly efficient removal efficiency (88.6 %) for ofloxacin (10 mg/L) degradation under wide pH ranges from 3.0 to 9.0. The evaluation of ecotoxicity, reusability, pH applicability, universality and anti-interference property confirmed the practical application prospect of nano-MnFe2O4-OH/PMS system.
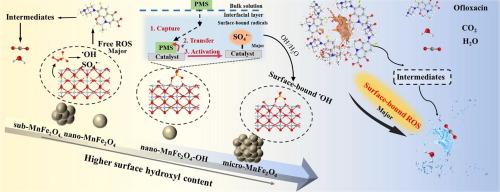
在 MnFe2O4 上将过硫酸氢钠的活化途径从自由基转换为表面结合自由基以增强氧氟沙星的降解:尺寸效应和表面羟基的关键作用
调节过一硫酸盐(PMS)活化途径以实现污染物的有效降解意义重大,但仍具有挑战性。本文通过碱性微环境调控策略合成了一系列羟基和尺寸可控的 MnFe2O4 催化剂。不同尺寸的 MnFe2O4(亚微米级、纳米级和微米级)表现出尺寸依赖性催化行为,而表面羟基含量的变化改变了从溶解自由基到表面结合自由基的活化途径。通过淬灭实验、电子自旋共振光谱、电化学研究、原位拉曼光谱和密度泛函理论计算,揭示了活性氧的演化过程。由于结合能很强,PMS 被丰富的表面羟基稳定,形成表面络合的 MnFe2O4-HOOSO3-,同时被活性双金属成分激活,从而定向产生表面键合自由基。得益于较小的粒径和丰富的羟基,最佳纳米-MnFe2O4-OH/PMS 体系能大量生成表面结合的 SO4--,在 3.0 到 9.0 的宽 pH 值范围内实现了高效的氧氟沙星(10 mg/L)降解去除率(88.6%)。生态毒性、可重复使用性、pH适用性、通用性和抗干扰性等方面的评价证实了纳米氧化锰铁氧体-OH/PMS系统的实际应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


