Synthesis of Highly Fluorescent Thiazole Fused Benzo[a] Carbazoles by Sunlight Driven Photocyclization of Indolylthiazoles
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein we report for the first time a sunlight‐driven, irreversible photocyclization reaction of indole‐linked trisubstituted thiazoles, for the synthesis of highly fluorescent thiazole‐fused benzo[
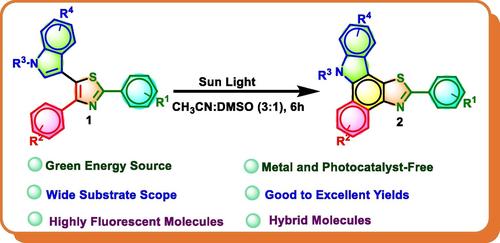
通过吲哚噻唑的日光驱动光环化合成高荧光噻唑融合苯并[a]咔唑
在此,我们首次报道了吲哚连接的三取代噻唑在阳光驱动下的不可逆光环化反应,该反应使用混合溶剂(CH3CN: DMSO; 3 : 1)合成了高荧光噻唑融合苯并[a]咔唑。在具有 2-甲基吲哚取代基的噻唑衍生物中观察到了吲哚分子的开环。在类似的反应条件下,以环状 1,3-二羰基取代吲哚基的三取代噻唑的光环化反应也起作用。该反应提供了具有噻唑和苯并咔唑两种重要药用分子的产物。我们研究了所有产物的光物理特性,发现大多数合成产物都具有非常好的荧光量子产率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


