Stoichiometry Dependence in the Consecutive, Competing Reduction, Halogenation, or Deoxygenation of Aryl Carbonyls
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Six fundamental chemical transformations of aryl carbonyls are achieved by properly adjusting the stoichiometry of the borane-amine and titanium tetrachloride reagent system. This set of reagents acts collectively as a hydride donor, Lewis acid catalyst, and halogen source for the reduction of carbonyls to alcohols, reductive halogenation of carbonyls to halides, deoxygenation of carbonyls to alkanes, dehydroxyhalogenation of alcohols to halides, deoxygenation of alcohols to alkanes, and hydrodehalogenation of halides to alkanes. While the carbonyl reduction is broadly applicable to both aromatic and aliphatic substrates, the remaining reactions are dependent on the stability of the proposed carbocationic intermediates, enabling highly selective reactions at the substrates’ benzylic position. This unique selectivity allows benzylic dehalogenation in the presence of aryl and alkyl halides in addition to highly selective dehydroxyhalogenation of alcohols even for tertiary versus secondary aliphatic and secondary versus primary benzylic substrates using only titanium tetrachloride as the chlorinating agent.
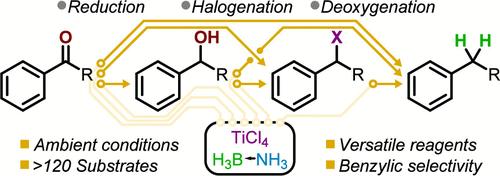
芳基羰基的连续、竞争还原、卤化或脱氧反应中的化学计量依赖性
通过适当调整硼烷-胺和四氯化钛试剂体系的化学计量,可以实现芳基羰基的六种基本化学转化。这组试剂共同充当氢化物供体、路易斯酸催化剂和卤素源,用于将羰基还原为醇、将羰基还原为卤化物、将羰基脱氧为烷、将醇脱氢卤化为卤化物、将醇脱氧为烷以及将卤化物脱氢卤化为烷。羰基还原反应广泛适用于芳香族和脂肪族底物,而其余反应则取决于所提议的碳代中间体的稳定性,从而在底物的苄基位置实现高选择性反应。这种独特的选择性允许在芳基和烷基卤化物存在的情况下进行苄基脱卤反应,以及对醇进行高选择性脱羟基卤化反应,即使只使用四氯化钛作为氯化剂,也可以对三级与二级脂肪族底物以及二级与一级苄基底物进行脱羟基卤化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


