A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
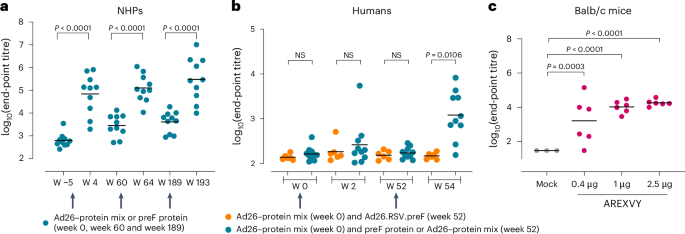
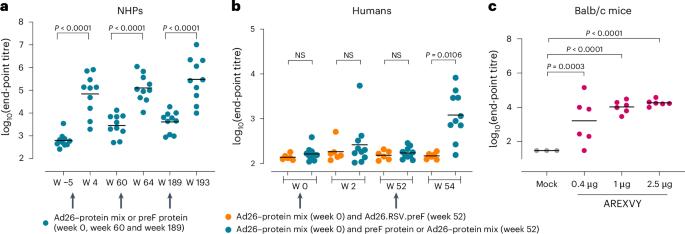
Respiratory syncytial virus (RSV) is a major cause of severe respiratory disease in infants and older people. Current RSV subunit vaccines are based on a fusion protein that is stabilized in the prefusion conformation and linked to a heterologous foldon trimerization domain to obtain a prefusion F (preF) trimer. Here we show that current RSV vaccines induce undesirable anti-foldon antibodies in non-human primates, mice and humans. To overcome this, we designed a foldon-free RSV preF trimer by elucidating the structural basis of trimerization-induced preF destabilization through molecular dynamics simulations and by introducing amino acid substitutions that negate hotspots of charge repulsion. The highly stable prefusion conformation was validated using antigenic and cryo-electron microscopy analysis. The preF is immunogenic and protective in naive mouse models and boosts neutralizing antibody titres in RSV-pre-exposed mice and non-human primates, while achieving similar titres to approved RSV vaccines in mice. This stable preF design is a promising option as a foldon-independent candidate for a next-generation RSV vaccine immunogen. A targeted design to stabilize the respiratory syncytial virus prefusion F trimer enables the generation of a foldon-free immunogen that elicits similar humoral responses to approved vaccines.
用于呼吸道合胞病毒的无折叠前灌注 F 三聚体疫苗,可减少脱靶免疫反应
呼吸道合胞病毒(RSV)是婴儿和老年人患严重呼吸道疾病的主要原因。目前的 RSV 亚单位疫苗基于一种融合蛋白,这种融合蛋白稳定在预融合构象中,并与异源折叠子三聚体结构域相连,从而获得预融合 F(preF)三聚体。在这里,我们发现目前的 RSV 疫苗会在非人灵长类动物、小鼠和人类体内诱发不良的抗折叠子抗体。为了克服这一问题,我们通过分子动力学模拟阐明了三聚体化引起的前F不稳定的结构基础,并通过引入氨基酸替代来消除电荷排斥热点,从而设计出了一种无折叠的 RSV 前F三聚体。抗原和冷冻电镜分析验证了高度稳定的前融合构象。该预融合构象在天真小鼠模型中具有免疫原性和保护性,并能提高暴露于 RSV 前的小鼠和非人灵长类动物的中和抗体滴度,同时在小鼠中达到与已获批准的 RSV 疫苗相似的滴度。这种稳定的前F设计是下一代RSV疫苗免疫原折叠独立候选物的理想选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


