Remodeling of Il4-Il13-Il5 locus underlies selective gene expression
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
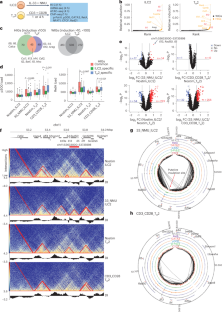
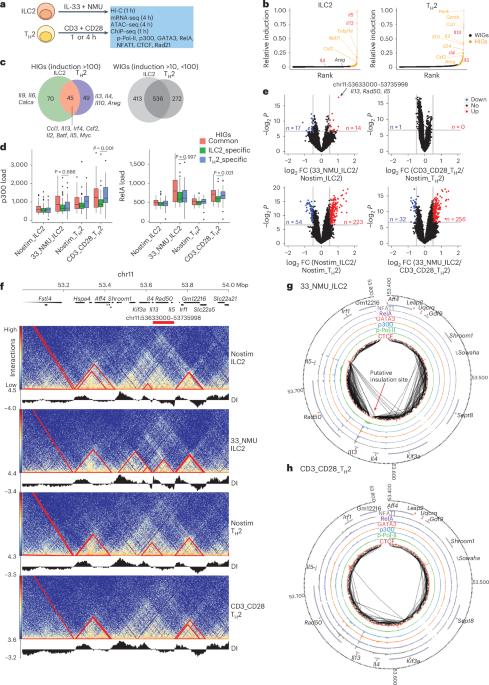
The type 2 cytokines, interleukin (IL)-4, IL-13 and IL-5 reside within a multigene cluster. Both innate (ILC2) and adaptive T helper 2 (TH2) lymphocytes secrete type 2 cytokines with diverse production spectra. Using transcription factor footprint and chromatin accessibility, we systemically cataloged regulatory elements (REs) denoted as SHS-I/II, KHS-I/II, +6.5kbIl13, 5HS-I(a, b, c, d, e), 5HS-II and 5HS-III(a, b, c) across the extended Il4-Il13-Il5 locus in mice. Physical proximities among REs were coordinately remodeled in three-dimensional space after cell activation, leading to divergent compartmentalization of Il4, Il13 and Il5 with varied combinations of REs. Deletions of REs revealed no single RE solely accounted for selective regulation of a given cytokine in vivo. Instead, individual RE differentially contribute to proper genomic positioning of REs and target genes. RE deletions resulted in context-dependent dysregulation of cytokine expression and immune response in tissue. Thus, signal-dependent remodeling of three-dimensional configuration underlies divergent cytokine outputs from the type 2 loci. O’Shea and colleagues examine the three-dimensional chromatin architecture of the type 2 cytokine locus and how it differs between innate ILC2 cells and adaptive TH2 lymphocytes.
Il4-Il13-Il5基因座重塑是选择性基因表达的基础
2 型细胞因子、白细胞介素 (IL)-4、IL-13 和 IL-5 位于一个多基因簇中。先天性(ILC2)和适应性 T 辅助细胞 2(TH2)淋巴细胞分泌的 2 型细胞因子具有不同的分泌谱。利用转录因子足迹和染色质可及性,我们对小鼠扩展的Il4-Il13-Il5基因座上的调控元件(Regulatory elements,REs)进行了系统编目,这些元件分别表示为SHS-I/II、KHS-I/II、+6.5kbIl13、5HS-I(a, b, c, d, e)、5HS-II和5HS-III(a, b, c)。细胞活化后,REs之间的物理邻近性在三维空间中发生了协调重塑,导致Il4、Il13和Il5在不同的REs组合下出现不同的区隔。REs的缺失表明,没有一个REs能单独对体内的特定细胞因子进行选择性调控。相反,单个RE对REs和靶基因的正确基因组定位有不同的贡献。RE缺失会导致细胞因子表达和组织免疫反应的环境依赖性失调。因此,信号依赖性三维构型重塑是 2 型基因座输出不同细胞因子的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


