Acute suppression of mitochondrial ATP production prevents apoptosis and provides an essential signal for NLRP3 inflammasome activation
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
How mitochondria reconcile roles in functionally divergent cell death pathways of apoptosis and NLRP3 inflammasome-mediated pyroptosis remains elusive, as is their precise role in NLRP3 activation and the evolutionarily conserved physiological function of NLRP3. Here, we have shown that when cells were challenged simultaneously, apoptosis was inhibited and NLRP3 activation prevailed. Apoptosis inhibition by structurally diverse NLRP3 activators, including nigericin, imiquimod, extracellular ATP, particles, and viruses, was not a consequence of inflammasome activation but rather of their effects on mitochondria. NLRP3 activators turned out as oxidative phosphorylation (OXPHOS) inhibitors, which we found to disrupt mitochondrial cristae architecture, leading to trapping of cytochrome c. Although this effect was alone not sufficient for NLRP3 activation, OXPHOS inhibitors became triggers of NLRP3 when combined with resiquimod or Yoda-1, suggesting that NLRP3 activation requires two simultaneous cellular signals, one of mitochondrial origin. Therefore, OXPHOS and apoptosis inhibition by NLRP3 activators provide stringency in cell death decisions.
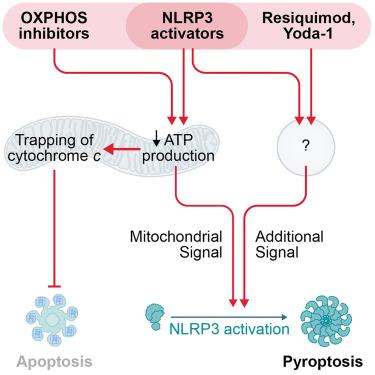
急性抑制线粒体 ATP 的产生可防止细胞凋亡,并为 NLRP3 炎症小体的激活提供重要信号
线粒体如何在细胞凋亡和 NLRP3 炎症体介导的热凋亡这两种功能不同的细胞死亡途径中协调作用,以及它们在 NLRP3 激活中的确切作用和 NLRP3 在进化过程中保守的生理功能,仍然是一个谜。在这里,我们证明了当细胞同时受到挑战时,细胞凋亡受到抑制,而 NLRP3 激活占优势。不同结构的 NLRP3 激活剂(包括尼革酸、咪喹莫特、细胞外 ATP、微粒和病毒)对细胞凋亡的抑制作用不是炎症小体激活的结果,而是它们对线粒体的影响。NLRP3 激活剂原来是氧化磷酸化(OXPHOS)抑制剂,我们发现它能破坏线粒体嵴的结构,导致细胞色素 c 的捕获。虽然这种效应本身不足以激活 NLRP3,但当 OXPHOS 抑制剂与雷司喹莫特或 Yoda-1 结合使用时,OXPHOS 抑制剂就成了 NLRP3 的触发器,这表明 NLRP3 的激活需要两个同时出现的细胞信号,其中一个来自线粒体。因此,NLRP3 激活剂对 OXPHOS 和细胞凋亡的抑制为细胞死亡决定提供了严格的条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


