Luminol/PtCo@rGO and Au@CNTs-based electrochemiluminescence cytosensor for ultrasensitive detection of breast cancer CTCs
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
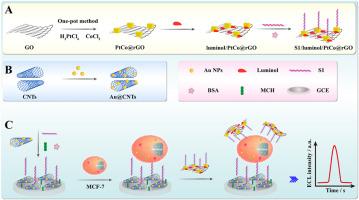
Breast cancer CTCs have recently been recognized as an emerging biomarker for liquid biopsy of breast cancer. In this work, based on two-dimensional (2D) noble metal PtCo@rGO nanozymes and Au@CNTs bioconjugates, a novel electrochemiluminescence (ECL) cytosensor was was developed in order to detect breast cancer CTCs (MCF-7) ultrasensitively.Results
The PtCo@rGO nanozymes possessed large specific surface area and high efficiency peroxidase-like activity, which can be used as nanocarriers to anchor and catalyze luminol ECL emission efficiently. Moreover, the PtCo@rGO nanozymes have fractal nanostructures similar to that of CTCs and can capable of enhancing the adhesion of MCF-7 when assembled together with aptamers containing HS-modified epithelial specific cell adhesion molecules (EpCAM, S1). Importantly, the S1/Au@CNTs bioconjugates loaded on the glassy carbon electrode (GCE) can effectively capture MCF-7 cells. Benefiting from the above-mentioned advantages, the ECL cytosensor constructed for MCF-7 cells detection performed well with a wide linear range (2 to 1×104 cells mL-1) and a low limit of detection (1 cells mL-1).Significance
The designed ECL cytosensor could provide a promising platform for CTC-based liquid biopsy and have broad application prospects in breast cancer early diagnosis and prognostic monitoring.
基于 Luminol/PtCo@rGO 和 Au@CNTs 的电化学发光细胞传感器用于超灵敏乳腺癌 CTCs 检测
背景最近,乳腺癌 CTCs 被认为是一种新兴的乳腺癌液体活检生物标记物。本研究基于二维贵金属 PtCo@rGO 纳米酶和 Au@CNTs 生物共轭物,开发了一种新型电化学发光(ECL)细胞传感器,用于超灵敏检测乳腺癌 CTCs(MCF-7)。此外,PtCo@rGO 纳米酶具有与四氯化碳类似的分形纳米结构,与含有 HS 修饰的上皮细胞特异性粘附分子(EpCAM,S1)的适配体组装在一起时,能够增强 MCF-7 的粘附性。重要的是,负载在玻璃碳电极(GCE)上的 S1/Au@CNTs 生物共轭物能有效捕获 MCF-7 细胞。得益于上述优点,用于 MCF-7 细胞检测的 ECL 细胞传感器性能良好,线性范围宽(2 至 1×104 cells mL-1),检测限低(1 cells mL-1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


