A pan-family screen of nuclear receptors in immunocytes reveals ligand-dependent inflammasome control
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Ligand-dependent transcription factors of the nuclear receptor (NR) family regulate diverse aspects of metazoan biology, enabling communications between distant organs via small lipophilic molecules. Here, we examined the impact of each of 35 NRs on differentiation and homeostatic maintenance of all major immunological cell types in vivo through a “Rainbow-CRISPR” screen. Receptors for retinoic acid exerted the most frequent cell-specific roles. NR requirements varied for resident macrophages of different tissues. Deletion of either Rxra or Rarg reduced frequencies of GATA6+ large peritoneal macrophages (LPMs). Retinoid X receptor alpha (RXRα) functioned conventionally by orchestrating LPM differentiation through chromatin and transcriptional regulation, whereas retinoic acid receptor gamma (RARγ) controlled LPM survival by regulating pyroptosis via association with the inflammasome adaptor ASC. RARγ antagonists activated caspases, and RARγ agonists inhibited cell death induced by several inflammasome activators. Our findings provide a broad view of NR function in the immune system and reveal a noncanonical role for a retinoid receptor in modulating inflammasome pathways.
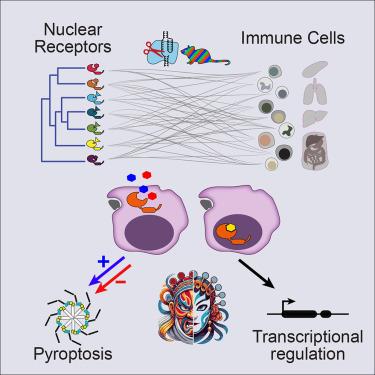
免疫细胞核受体泛家族筛选揭示配体依赖性炎症小体控制
核受体(NR)家族的配体依赖性转录因子调控着类人猿生物学的各个方面,通过亲脂性小分子实现远距离器官之间的通讯。在这里,我们通过 "彩虹-CRISPR "筛选研究了 35 种 NR 对体内所有主要免疫细胞类型的分化和平衡维持的影响。视黄酸受体发挥了最常见的细胞特异性作用。不同组织的常驻巨噬细胞对 NR 的需求各不相同。Rxra或Rarg的缺失会降低GATA6+大腹腔巨噬细胞(LPMs)的频率。视黄酸 X 受体α(RXRα)的传统功能是通过染色质和转录调控来协调 LPM 的分化,而视黄酸受体γ(RARγ)则通过与炎性体适配体 ASC 的结合来调控热凋亡,从而控制 LPM 的存活。RARγ拮抗剂可激活caspases,而RARγ激动剂可抑制几种炎症小体激活剂诱导的细胞死亡。我们的发现为 NR 在免疫系统中的功能提供了一个广阔的视角,并揭示了视黄醇受体在调节炎性体通路中的非经典作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


