α-Nitrocinnamic Nitriles in Reactions with 1-Methylpyrrole, Hydrazine, Phenylhydrazine, and o-Aminothiophenol
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reaction of unsubstituted α-nitrocinnamic nitrile with 1-methylpyrrole ends with the formation of an AdN product, whereas the reactions of 2-aryl-2-nitroacrylonitriles with such N,N- and N,S-binucleophiles as hydrazine, phenylhydrazine, and o-aminothiophenol lead to transformation products of the initially formed adducts, linear azines and phenylhydrazones, or to a heterocyclic benzothiazole structure.
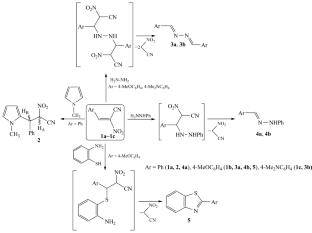
α-硝基肉桂腈与 1-甲基吡咯、肼、苯肼和邻氨基苯硫酚的反应
未取代的 α-硝基肉桂腈与 1-甲基吡咯的反应以形成 AdN 产物而告终,而 2-芳基-2-硝基丙烯腈与 N,N-和 N,S-亲核剂(如肼、苯肼和邻氨基苯硫酚)的反应则导致最初形成的加合物、线型叠氮和苯肼或杂环苯并噻唑结构的转化产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


