Modulating NH3 oxidation and inhibiting sulfate deposition to improve NH3-SCR denitration performance by controlling Mn/Nb ratio over MnaNbTi2Ox (a = 0.6-0.9) catalysts
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The MnaNbTi2Ox (a = 0.6–0.9) catalysts for NH3 selective catalytic reduction denitration were prepared using the co-precipitation method. Among them, Mn0.7NbTi2Ox exhibits well low-temperature catalytic performance, wide activity temperature range (180–480 ℃), and worthy resistance to SO2 even with H2O. XRD was used to investigate the structure of MnaNbTi2Ox, in which Mn and Nb oxides highly dispersed in the MnaNbTi2Ox catalysts and Nb can dope into the crystal lattice of TiO2. XRF and XPS results show Nb can affect the transfer of electrons to Mn4+, and changing the Mn/Nb ratio can regulate the Mn4+ content on the MnaNbTi2Ox catalysts. H2-TPR, NH3 and NO oxidation results verify that Nb inhibits the oxidation capacity of MnaNbTi2Ox, and altering the Mn/Nb ratio can get appropriate oxidation property, which facilitates low-temperature NH3 activation and limit non-selective oxidation for NH3. In-situ DRIFTS results show Nb-OH bonds can provide new Brønsted acid sites, and both Lewis and Brønsted acid sites are active. Furthermore, Nb addition prevents sulphate deposition on the catalyst. The effect of Mn/Nb on catalytic performance, N2O formation and inhibition, SO2 poisoning, SO2 effect on NH3-SCR, and the enhancement of SO2 tolerance are also analyzed.
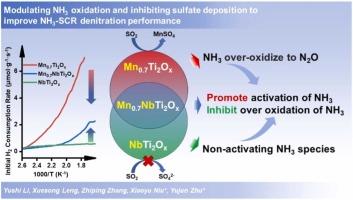
通过控制 MnaNbTi2Ox(a = 0.6-0.9)催化剂上的锰/铌比来调节 NH3 氧化和抑制硫酸盐沉积,从而提高 NH3-SCR 脱硝性能
采用共沉淀法制备了用于 NH3 选择性催化还原脱硝的 MnaNbTi2Ox(a = 0.6-0.9)催化剂。其中,Mn0.7NbTi2Ox 具有良好的低温催化性能、较宽的活性温度范围(180-480 ℃)以及即使在有 H2O 的情况下也能抵抗 SO2 的特性。XRD 被用来研究 MnaNbTi2Ox 的结构,其中 Mn 和 Nb 氧化物高度分散在 MnaNbTi2Ox 催化剂中,Nb 可以掺杂到 TiO2 的晶格中。XRF 和 XPS 结果表明,Nb 可以影响电子向 Mn4+ 的转移,改变 Mn/Nb 的比例可以调节 MnaNbTi2Ox 催化剂上 Mn4+ 的含量。H2-TPR、NH3 和 NO 氧化结果验证了 Nb 会抑制 MnaNbTi2Ox 的氧化能力,而改变 Mn/Nb 的比例可以获得适当的氧化性能,从而促进 NH3 的低温活化并限制 NH3 的非选择性氧化。原位 DRIFTS 结果表明,Nb-OH 键可以提供新的布氏酸位点,而且路易斯酸位点和布氏酸位点都很活跃。此外,铌的添加还能防止硫酸盐在催化剂上沉积。此外,还分析了 Mn/Nb 对催化性能、N2O 生成和抑制、二氧化硫中毒、二氧化硫对 NH3-SCR 的影响以及二氧化硫耐受性的增强等方面的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


