Discovery of potent and selective factor XIa inhibitors incorporating triazole-based benzoic acid as novel P2’ fragments: Molecular dynamics simulations and anticoagulant activity
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Factor XIa (FXIa) has emerged as a novel anticoagulant target with a reduced risk of bleeding. However, due to the nearly identical residues it shares with its closest homologue, plasma kallikrein (PKa), only a few selective FXIa inhibitors have been reported. Herein, we describe the discovery of novel triazole-based pyridone derivatives as potent and selective FXIa inhibitors. Structural optimization identified triazole-based benzoic acids as optimal P2′ fragments. The representative compound (S)-10h (IC50 = 0.38 nM for FXIa) was approximately 3-fold more potent than asundexian for FXIa, along with up to 150-fold selectivity over PKa (13-fold for asundexian) and up to 100,000-fold selectivity over FXa and thrombin (5000-fold for asundexian). Extensive molecular dynamics simulations and free energy calculations revealed that electrostatic interactions with varied residues near the binding site, particularly the loop at the bottom of the S2’ pocket (IP-loop), are critical factors contributing to the improved selectivity over PKa. Calculations of electrostatic potential (ESP) surfaces illustrated that FXIa forms a more positive ESP than PKa, thrombin, and FXa, which attracts the carboxylic acid group of the designed compounds, enhancing both potency and selectivity. Moreover, compound (S)-10h demonstrated potent in vitro anticoagulant activity with an EC1.5X value of 0.55 μM for aPTT, without interfering with PT up to 100 μM. Thus, compound (S)-10h represents a promising lead for further optimization as a novel anticoagulant agent.
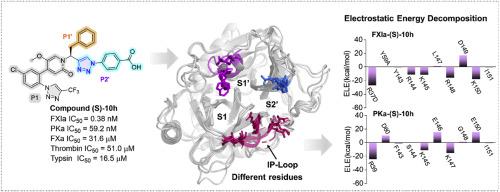
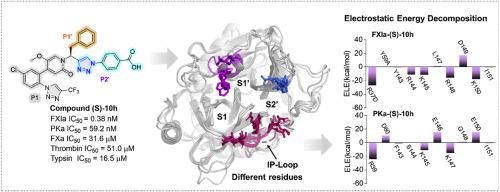
发现以三唑基苯甲酸为新型 P2'片段的强效和选择性因子 XIa 抑制剂:分子动力学模拟与抗凝活性
因子 XIa(FXIa)已成为一种可降低出血风险的新型抗凝血靶点。然而,由于它与其最接近的同源物血浆钙激酶(PKa)具有几乎相同的残基,目前只报道了少数几种选择性 FXIa 抑制剂。在此,我们介绍了新型三唑基吡啶酮衍生物作为强效选择性 FXIa 抑制剂的发现。结构优化确定三唑基苯甲酸为最佳 P2'片段。代表性化合物 (S)-10h(对 FXIa 的 IC50 = 0.38 nM)对 FXIa 的作用比 asundexian 强约 3 倍,对 PKa 的选择性高达 150 倍(asundexian 为 13 倍),对 FXa 和凝血酶的选择性高达 100,000 倍(asundexian 为 5,000 倍)。广泛的分子动力学模拟和自由能计算显示,与结合位点附近不同残基的静电相互作用,尤其是 S2' 口袋底部的环路(IP-loop),是提高对 PKa 选择性的关键因素。静电位(ESP)表面的计算结果表明,FXIa 形成的 ESP 比 PKa、凝血酶和 FXa 更正,从而吸引了所设计化合物的羧基,提高了药效和选择性。此外,(S)-10h 化合物显示出了强大的体外抗凝活性,对 aPTT 的 EC1.5X 值为 0.55 μM,而对 PT 的干扰不超过 100 μM。因此,(S)-10h 化合物是一种有望进一步优化的新型抗凝剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


