Electrochemical CO2 reduction chemistry of C1 and C2+ products on Cu/Zn electrodes via galvanic replacement
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrochemical (EC) reduction of CO2 has gained significant interest for producing value-added products, especially with Cu-based electrodes. In this study, a Cu/Zn electrode was prepared via galvanic replacement and evaluated for its efficiency in generating C1 and C2+ products during EC CO2 reduction. Key experimental parameters included applied potentials, electrolyte concentrations, light irradiation, and electrode configurations. The Cu/Zn electrode demonstrated notably high selectivity for ethanol, alongside syngas (CO and H2) production. The formation of ethanol and CO was primarily influenced by the applied potential and electrolyte concentration. Post-reaction analysis revealed substantial changes in the electrode's morphology, crystal structure, oxidation states, and Cu/Zn ratios. These findings enhanced the understanding of ethanol production mechanisms and C1/C2+ product formation, contributing to the development of more effective bimetallic electrodes for CO2 reduction.
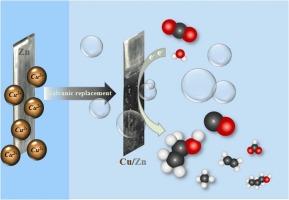
通过电化学置换实现 Cu/Zn 电极上 C1 和 C2+ 产物的电化学 CO2 还原化学反应
二氧化碳的电化学(EC)还原在生产高附加值产品方面获得了极大的关注,尤其是使用铜基电极。本研究通过电镀置换法制备了铜/锌电极,并评估了其在电化学还原二氧化碳过程中生成 C1 和 C2+ 产物的效率。主要实验参数包括应用电位、电解质浓度、光照射和电极配置。Cu/Zn 电极在产生合成气(CO 和 H2)的同时,对乙醇的选择性也很高。乙醇和 CO 的生成主要受应用电位和电解质浓度的影响。反应后分析表明,电极的形态、晶体结构、氧化态和铜/锌比例发生了很大变化。这些发现加深了人们对乙醇生成机制和 C1/C2+ 产物形成的理解,有助于开发出更有效的双金属电极来还原 CO2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


