Design of cellulose/polyethylene oxide/EMITFSI-based composite electrolyte with synergistic transport mechanism for high-performance solid-state lithium batteries
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Despite its theoretically high energy density, polymer solid-state lithium batteries (PSSLBs) exhibit lower actual energy density. This discrepancy arises from the low ionic conductivity of the polymer solid-state electrolyte (PSSE) due to the coupling of lithium ion (Li+) transport to the relaxation of polymer chain segments. The objective of this study is to optimize the Li+ transport in PSSE. This is achieved by incorporating 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMITFSI) to plasticize both cellulose and polyethylene oxide (PEO). By leveraging the synergistic effects of cellulose and PEO, an ion-conducting network is established. This network allows Li+ to form multiple Li-O coordination simultaneously with the hydroxyl group (OH) of cellulose and the ether group (EO) of PEO, thereby enabling Li+ to transport between the two polymers in a decoupled manner. The PSSE demonstrated an ionic conductivity of 4 × 10-4 mS/cm (at room temperature) and a Li+ transference number of 0.43, significantly exceeding traditional PEO-based values of 10-5 mS/cm and 0.1–0.2. Additionally, the high voltage stability of EMITFSI extends the electrochemical stability window of PSSE, achieving a stability window of 5 V. The assembled LiFePO4/Li cell achieved a specific capacity of 138 mA h/g at 50℃ (0.5C) with a capacity retention rate of 80 % after 280 cycles. This represents an innovative method for preparing high-energy–density solid-state lithium batteries.
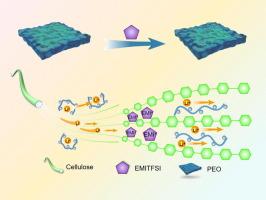
为高性能固态锂电池设计具有协同传输机制的纤维素/聚环氧乙烷/EMITFSI 基复合电解质
尽管聚合物固态锂电池(PSSLB)的理论能量密度很高,但其实际能量密度却较低。造成这种差异的原因是聚合物固态电解质(PSSE)的离子传导性较低,这是由于锂离子(Li+)传输与聚合物链段的弛豫耦合造成的。本研究的目的是优化 PSSE 中的 Li+ 传输。其方法是将 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺(EMITFSI)用于纤维素和聚氧化乙烯(PEO)的塑化。利用纤维素和聚环氧乙烷的协同效应,可以建立一个离子传导网络。这种网络可使 Li+ 同时与纤维素的羟基(OH)和 PEO 的醚基(EO)形成多个 Li-O 配位,从而使 Li+ 以解耦方式在两种聚合物之间传输。PSSE 的离子电导率为 4 × 10-4 mS/cm(室温下),Li+转移数为 0.43,大大超过了基于 PEO 的 10-5 mS/cm 和 0.1-0.2 的传统值。此外,EMITFSI 的高电压稳定性扩展了 PSSE 的电化学稳定性窗口,实现了 5 V 的稳定性窗口。组装后的 LiFePO4/Li 电池在 50℃(0.5C)条件下的比容量为 138 mA h/g,280 个循环后的容量保持率为 80%。这是制备高能量密度固态锂电池的一种创新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


