Convenient synthesis of granulated Li/Al-layered double hydroxides/chitosan composite adsorbents for lithium extraction from simulated brine with a high Mg2+/Li+ ratio
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
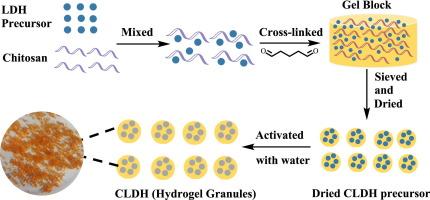
Lithium/aluminum layered double hydroxides (LDH) have been industrially applied to extract lithium from salt-lake brine. Different from traditional granulation methods, chitosan with high hydrophilicity was used as the binder of LDH in this work, and LDH/chitosan composite hydrogel granules (CLDH) with a diameter of about 1–2 mm were successfully prepared. The new granulation method is simple, cost-effective, and does not require the use of organic solvents. The chemical composition and structure of CLDH were characterized by techniques such as FTIR, XRD, FESEM, TEM, TGA, and XPS. In simulated brine with a Mg2+/Li+ ratio of 102, the maximum saturated adsorption capacity of lithium on CLDH-3 reached 12.5 mg g−1 (pH = 6.5), but it took 24 h to reach adsorption equilibrium. During the three successive cycles for the extraction of Li+ from simulated brine by the CLDH-3 column at the flow rate of 1 mL min−1, the adsorption capacities of Li+ on CLDH-3 in the three cycles were 15.4, 15.9 and 12.1 mg g−1, respectively. Moreover, deionized water (30 °C) was used as the eluent to recover lithium adsorbed on the CLDH-3 column, and the lithium recovery efficiencies reached 50.7 %, 53.6 %, and 62.8 % of the column adsorption capacities over the three cycles, respectively, and the Mg/Li ratios in the eluents were 0.47, 0.71, and 0.50, respectively. Overall, CLDH-3 exhibits good reusability, mechanical strength, structural stability, and selectivity in the process of lithium extraction from simulated salt-lake brine, and has potential industrial application value.
方便合成颗粒状锂/铝层双氢氧化物/壳聚糖复合吸附剂,用于从高 Mg2+/Li+ 比的模拟盐水中提取锂
锂/铝层状双氢氧化物(LDH)已被工业化应用于从盐湖卤水中提取锂。与传统造粒方法不同,本研究采用亲水性较强的壳聚糖作为 LDH 的粘合剂,成功制备出直径约为 1-2 毫米的 LDH/ 壳聚糖复合水凝胶颗粒(CLDH)。新的制粒方法简单、成本低廉,且无需使用有机溶剂。傅立叶变换红外光谱、X射线衍射、FESEM、TEM、TGA和XPS等技术对CLDH的化学成分和结构进行了表征。在 Mg2+/Li+ 比率为 102 的模拟盐水中,CLDH-3 对锂的最大饱和吸附容量达到 12.5 mg g-1(pH = 6.5),但需要 24 小时才能达到吸附平衡。在流量为 1 mL min-1 的条件下,CLDH-3 柱连续三次从模拟盐水中萃取 Li+,三次循环中 CLDH-3 柱对 Li+的吸附容量分别为 15.4、15.9 和 12.1 mg g-1。此外,用去离子水(30 °C)作为洗脱液来回收 CLDH-3 柱上吸附的锂,三个周期的锂回收率分别达到柱吸附容量的 50.7%、53.6% 和 62.8%,洗脱液中的镁/锂比分别为 0.47、0.71 和 0.50。总体而言,CLDH-3 在模拟盐湖卤水提锂过程中表现出良好的重复使用性、机械强度、结构稳定性和选择性,具有潜在的工业应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
文献相关原料
公司名称
产品信息
阿拉丁
Aluminum chloride hexahydrate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


