In(OTf)3-Catalyzed (3 + 3) Dipolar Cyclization of Bicyclo[1.1.0]butanes with N-Nucleophilic 1,3-Dipoles: Access to 2,3-Diazabicyclo[3.1.1]heptanes, 2,3-Diazabicyclo[3.1.1]heptenes, and Enantiopure 2-Azabicyclo[3.1.1]heptanes
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The investigation into the synthesis of azabicyclo[3.1.1]heptanes (azaBCHeps) as bioisosteres to flat aza-aromatics has garnered increasing attention, while it encounters significant challenges. Herein, we have demonstrated the In(OTf)3-catalyzed (3 + 3) dipolar cyclization of bicyclo[1.1.0]butanes (BCBs) with hydrazones and π-allyl-iridium 1,3-dipoles, engendering a diverse array of azaBCHeps. The cyclization of hydrazones and BCBs furnished densely substituted 2,3-diazabicyclo[3.1.1]heptanes and 2,3-diazabicyclo[3.1.1]heptenes under nitrogen and oxygen atmospheres, respectively. A combination of experimental and computational investigations lends robust support for the proton-transfer-interposed sequential mechanism. More importantly, by integrating In(OTf)3/iridium relay catalysis, enantiopure 2-azabicyclo[3.1.1]heptanes were constructed through the (3 + 3) cyclization of BCBs with aza-π-allyl-iridium 1,3-dipoles, in situ generated from N-allyl carbonates. Both methodologies exhibit mild reaction conditions and good tolerance for various functional groups. Moreover, the copious derivatization of products highlights the utility of the newly synthesized heterobicyclic motifs as versatile building blocks in synthetic chemistry.
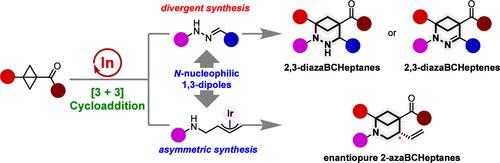
In(OTf)3-Catalyzed (3 + 3) Dipolar Cyclization of Bicyclo[1.1.0]butanes with N-Nucleophilic 1,3-Dipoles:获得 2,3-二氮杂双环[3.1.1]庚烷、2,3-二氮杂双环[3.1.1]庚烯和不纯的 2-Azabicyclo[3.1.1]heptanes
氮杂双环[3.1.1]庚烷(azabicyclo[3.1.1]heptanes,azBCHeps)作为生物异构体合成平氮杂芳烃的研究越来越受到关注,同时也遇到了巨大的挑战。在此,我们展示了 In(OTf)3 催化的双环[1.1.0]丁烷(BCBs)与肼酮和π-烯丙基铱 1,3- 二极的 (3 + 3) 双极环化反应,从而产生了一系列不同的 azaBCHeps。在氮气和氧气气氛下,肼和 BCB 的环化反应分别生成了高取代度的 2,3- 二氮杂双环[3.1.1]庚烷和 2,3- 二氮杂双环[3.1.1]庚烯。实验和计算研究的结合为质子转移叠加顺序机制提供了有力的支持。更重要的是,通过整合 In(OTf)3/iridium 中继催化,以 N-烯丙基碳酸盐为原位生成的杂氮-π-烯丙基-1,3-铱二极体与 BCBs 发生 (3 + 3) 环化反应,构建了对映体纯的 2-氮杂双环[3.1.1]庚烷。这两种方法的反应条件温和,对各种官能团具有良好的耐受性。此外,产物的大量衍生化凸显了新合成的杂双环基团在合成化学中作为多功能构建模块的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


