A top-down slow breathing circuit that alleviates negative affect in mice
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
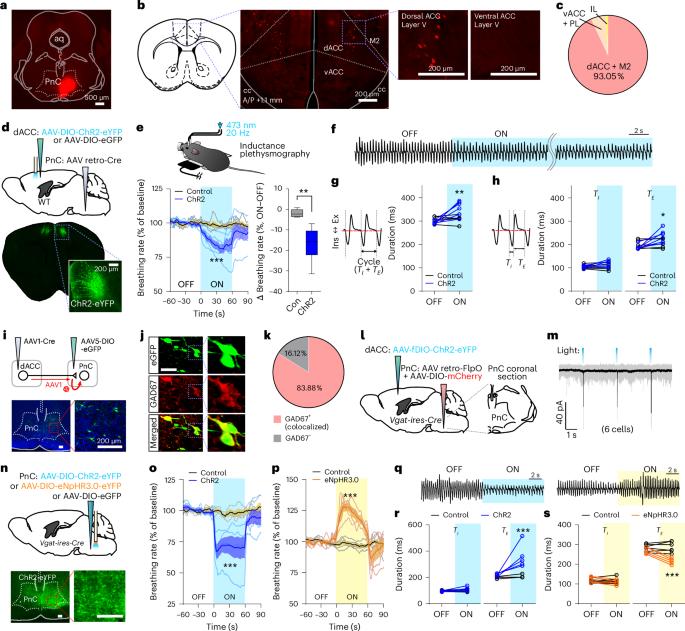
Although breathing is primarily automatic, its modulation by behavior and emotions suggests cortical inputs to brainstem respiratory networks, which hitherto have received little characterization. Here we identify in mice a top-down breathing pathway from dorsal anterior cingulate cortex (dACC) neurons to pontine reticular nucleus GABAergic inhibitory neurons (PnCGABA), which then project to the ventrolateral medulla (VLM). dACC→PnC activity correlates with slow breathing cycles and volitional orofacial behaviors and is influenced by anxiogenic conditions. Optogenetic stimulation of the dACC→PnCGABA→VLM circuit simultaneously slows breathing and suppresses anxiety-like behaviors, whereas optogenetic inhibition increases both breathing rate and anxiety-like behaviors. These findings suggest that the dACC→PnCGABA→VLM circuit has a crucial role in coordinating slow breathing and reducing negative affect. Our study elucidates a circuit basis for top-down control of breathing, which can influence emotional states. Jhang et al. identify a prefrontal–pontomedullary pathway that slows breathing and reduces anxiety in mice, where the pontine reticular nucleus converts excitatory prefrontal inputs into inhibitory signals to brainstem respiratory networks.
缓解小鼠负面情绪的自上而下缓慢呼吸回路
虽然呼吸主要是自动的,但它受行为和情绪的调控,这表明大脑皮层对脑干呼吸网络有输入作用,而迄今为止,大脑皮层对呼吸网络的描述还很少。在这里,我们发现了小鼠从背侧前扣带回皮层(dACC)神经元到桥脑网状核GABA能抑制神经元(PnCGABA)的自上而下的呼吸通路,该通路随后投射到腹外侧延髓(VLM)。对dACC→PnCGABA→VLM回路的光遗传刺激可同时减慢呼吸和抑制焦虑样行为,而光遗传抑制可同时增加呼吸频率和焦虑样行为。这些发现表明,dACC→PnCGABA→VLM回路在协调缓慢呼吸和减少负性情绪方面起着至关重要的作用。我们的研究阐明了自上而下控制呼吸的回路基础,而呼吸可以影响情绪状态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


