A Ternary Bio-Based Monomer toward the Polyesters with High UV Shielding and Water-Degradation Properties
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Developing renewable monomers for preparing polyesters with high UV shielding and hydrolytic degradability is a challenging topic. Herein, a ternary cyclic monomer (denoted as FBPC) containing one furan and two pyrrolidones was prepared by an aza-Michael addition reaction using biobased furan diamine and dimethyl itaconate (DMI). FBPC was melt polymerized with various diols to prepare homopolyesters with a number-average molecular mass (Mn) in the range of 22.4–30.3 kDa. The homopolyesters based on FBPC presented excellent UV shielding properties, with a maximum shielding cutoff of 398 nm, which is significantly superior to monofuran-based polyesters, such as poly(ethylene furanoate) (PEF) and poly(methyl 5-[(2-hydroxyethyl)-sulfanyl]furan-2-carboxylate) (pMSF). The hydrophilic pyrrolidone rings in FBPC enhance the hydrolytic sensitivity of the homopolyesters, giving them complete degradation within 130 days. Then, FBPC was copolymerized with poly(butylene terephthalate) (PBT) to prepare a series of copolyesters with Mn of 23.9–42.7 kDa. The UV shielding and hydrolytic degradation of PBT were significantly improved by adding a ternary cyclic monomer. In addition, FBPC was effective in toughening PBT without changing the thermal stability, and the toughness effect far exceeded those of other sugar-derived cyclic monomers. The mechanical, UV shielding, and hydrolytic degradation properties of the copolyesters can be adjusted depending on FBPC content. Overall, FBPC is an effective biobased precursor that can offer new solutions for improving polyester properties, including UV shielding and hydrolytic degradation.
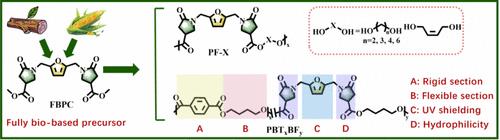
具有高紫外线屏蔽和水降解性能的三元生物基单体聚酯
开发用于制备具有高紫外线屏蔽和水解降解性的聚酯的可再生单体是一个具有挑战性的课题。在此,我们使用生物基呋喃二胺和衣康酸二甲酯(DMI)通过氮杂迈克尔加成反应制备了一种含有一个呋喃和两个吡咯烷酮的三元环状单体(称为 FBPC)。FBPC 与各种二元醇进行熔融聚合,制备出平均分子质量 (Mn) 在 22.4-30.3 kDa 范围内的均聚酯。基于 FBPC 的均聚酯具有优异的紫外线屏蔽性能,最大屏蔽截止波长为 398 纳米,明显优于单呋喃基聚酯,如聚(呋喃乙烯酯)(PEF)和聚(5-[(2-羟乙基)-硫]呋喃-2-甲酸甲酯)(pMSF)。FBPC 中的亲水性吡咯烷酮环增强了均聚物的水解敏感性,使其在 130 天内完全降解。然后,将 FBPC 与聚(对苯二甲酸丁二醇酯)(PBT)共聚,制备出一系列 Mn 为 23.9-42.7 kDa 的共聚物。通过添加三元环状单体,PBT 的紫外线屏蔽和水解降解性能得到了显著改善。此外,FBPC 还能在不改变热稳定性的情况下有效增韧 PBT,其增韧效果远远超过了其他糖源环状单体。共聚聚酯的机械、紫外线屏蔽和水解降解性能可根据 FBPC 的含量进行调整。总之,FBPC 是一种有效的生物基前体,可为改善聚酯性能(包括紫外线屏蔽和水解降解性能)提供新的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


