Impact of Lithium Nonstoichiometry on Ionic Diffusion in Tetragonal Garnet-Type Li7La3Zr2O12
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
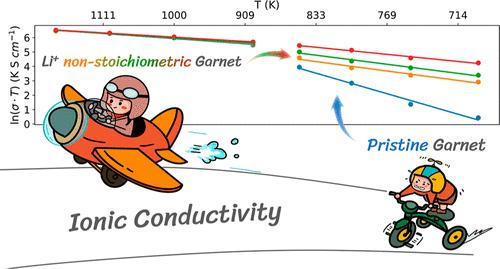
Understanding ion transport mechanisms on the atomistic scale in solid-state electrolytes is crucial for the development of all-solid-state batteries. Li7La3Zr2O12 (LLZO) is a promising oxide solid electrolyte material, whose phase transition behavior and ion transport mechanisms have attracted significant research attention. Previous studies have primarily focused on ion transport in the cubic phase (intrinsic high-temperature phase or doped variants). In contrast, the tetragonal phase of LLZO, despite its close relationship with the cubic phase, has received less attention due to its relatively low ionic conductivity and high computational cost. A few recent computational studies have shown significant discrepancies in conductivity and activation energy between calculated and experimental values. Therefore, the unclear ion transport mechanisms in the tetragonal phase of LLZO are critical to understanding and designing oxide solid electrolytes. In this study, we employ state-of-the-art machine-learning-based neuroevolution potential molecular dynamics simulations to investigate the effects of lithium nonstoichiometry on the ionic conductivity and phase stability of LLZO. We demonstrate that small deviations from stoichiometry, particularly lithium deficiency, dramatically reduce the activation energy for Li+ diffusion in tetragonal LLZO from 1.227 to 0.425 eV, increasing room-temperature ionic conductivity by 10 orders of magnitude. The slight lithium nonstoichiometry, which commonly occurs during high-temperature synthesis, has a significant effect on ion transport in the tetragonal phase. Our findings highlight the crucial role of lithium nonstoichiometry and defect chemistry in enhancing LLZO performance and provide insights for the rational design of high-performance solid electrolytes through defect engineering.
锂的不均匀性对四方石榴石型 Li7La3Zr2O12 中离子扩散的影响
了解固态电解质中原子尺度的离子传输机制对于开发全固态电池至关重要。Li7La3Zr2O12(LLZO)是一种很有前途的氧化物固态电解质材料,其相变行为和离子传输机制已引起了大量研究的关注。以往的研究主要集中于立方相(固有高温相或掺杂变体)的离子传输。相比之下,尽管 LLZO 的四方相与立方相关系密切,但由于其离子电导率相对较低且计算成本较高,因此受到的关注较少。最近的一些计算研究表明,计算值和实验值在电导率和活化能方面存在显著差异。因此,弄清 LLZO 四方相中的离子传输机制对于理解和设计氧化物固体电解质至关重要。在本研究中,我们采用了最先进的基于机器学习的神经进化势分子动力学模拟来研究锂的非几何形状对 LLZO 离子电导率和相稳定性的影响。我们证明,与化学计量的微小偏差,尤其是锂的缺乏,会将四方 LLZO 中 Li+ 扩散的活化能从 1.227 电子伏特大幅降低到 0.425 电子伏特,从而将室温离子电导率提高 10 个数量级。在高温合成过程中通常会出现轻微的锂非全度性,这对四方相中的离子传输有显著影响。我们的研究结果凸显了锂非全度性和缺陷化学在提高 LLZO 性能方面的关键作用,并为通过缺陷工程合理设计高性能固体电解质提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


