Enhancing bone repair efficiency through synergistic modification of recombinant human collagen onto PLLA membranes
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-12-01
DOI:10.1016/j.ijbiomac.2024.137631
引用次数: 0
Abstract
Given the exponential growth of the recombinant human collagen market, it is paramount to devise a robust and straightforward design strategy aimed at preserving the remarkable biological activity of recombinant human collagen while endowing it with tailored mechanical properties and stable morphologies. This innovative approach stands to broaden its applicability in hard tissue repair endeavors. Our study employed a synergistic approach of alkali hydrolysis and Schiff's base chemistry to graft Type I recombinant human collagen (rhCol-I) onto poly (L-lactic acid) (PLLA) membranes, yielding PLLA-rhCol composites. In vitro evaluations substantiated that this reengineered material not only retained the biological efficacy of rhCol-I but also imparted mechanical robustness and processability ideal for bone implant applications. Notably, it exhibited superior tissue engineering attributes, fostering proliferation, adhesion, osteogenic differentiation, mineralization of bone marrow mesenchymal stem cells (BMSCs), and encouraging vascularization. In a rat model of critical-sized bone defects, PLLA-rhCol exhibited markedly enhanced bone repair efficiency over conventional PLLA bone implants, achieving a bone volume fraction (BV/TV) of up to 32.57 ± 3.77 %, while promoting angiogenesis and effectively mitigating inflammatory cell infiltration. This pioneering method of modifying recombinant human collagen onto the side chains of polymeric macromolecules portends broad applicability in enhancing various biocompatible, yet mechanically robust and processable polymers, thereby expanding the horizons of recombinant human collagen utilization in tissue engineering and catering to the ever-evolving market demands.
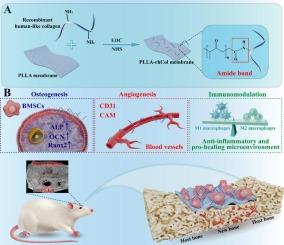
通过对聚乳酸膜上的重组人胶原蛋白进行协同改性,提高骨修复效率。
鉴于重组人胶原蛋白市场的指数式增长,最重要的是设计出一种稳健而直接的设计策略,旨在保留重组人胶原蛋白的显著生物活性,同时赋予其量身定制的机械性能和稳定形态。这种创新方法将拓宽其在硬组织修复领域的应用范围。我们的研究采用碱水解和希夫碱化学的协同方法,将 I 型重组人胶原蛋白(rhCol-I)接枝到聚(L-乳酸)(PLLA)膜上,形成 PLLA-rhCol 复合材料。体外评估证实,这种重新设计的材料不仅保留了 rhCol-I 的生物功效,还具有机械坚固性和可加工性,是骨植入应用的理想材料。值得注意的是,它表现出卓越的组织工程特性,促进了骨髓间充质干细胞(BMSCs)的增殖、粘附、成骨分化和矿化,并促进了血管化。在大鼠临界大小骨缺损模型中,PLLA-rhCol 的骨修复效率明显高于传统 PLLA 骨植入物,骨体积分数(BV/TV)高达 32.57 ± 3.77 %,同时还能促进血管生成,有效减轻炎症细胞浸润。这种将重组人胶原蛋白改性到聚合物大分子侧链上的开创性方法可广泛应用于增强各种生物相容性、机械坚固性和可加工性的聚合物,从而扩大重组人胶原蛋白在组织工程中的应用范围,满足不断发展的市场需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


