A State-of-the-Art Review on the Recent Advances in Exosomes in Oncogenic Virus
Abstract
Background and Aims
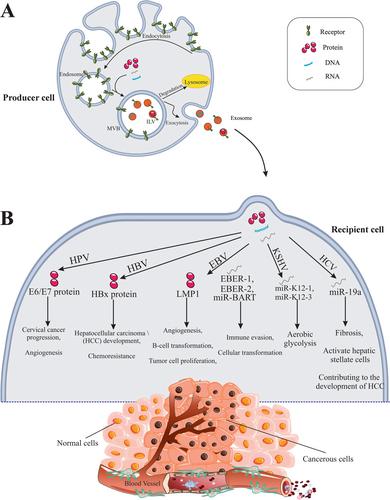
Oncogenic viruses are responsible for approximately 12% of human malignancies, influencing various cancer processes through intricate interactions with host cells. Exosomes (EXOs), nanometric-sized microvesicles involved in cell communication, have emerged as critical mediators in these interactions. This review aims to explore the mechanisms by which EXOs produced by cells infected with oncogenic viruses promote cancer growth, enhance viral transmissibility, and act as immunomodulators.
Methods
A comprehensive review was conducted, focusing on recent studies highlighting the mechanisms by which EXOs facilitate the oncogenic potential of viruses. The analysis included the characterization of exosomal content, such as microRNAs (miRNAs) and proteins, and their effects on tumor microenvironments and immune responses. A search was performed using databases including PubMed, ScienceDirect, and Google Scholar. MeSH keywords related to EXOs, oncogenic viruses, and cancer were used to retrieve relevant review, systematic, and research articles.
Results
Findings indicate that EXOs from oncogenic virus-infected cells carry viral components that facilitate infection and inflammation. These EXOs alter the tumor microenvironment, contributing to the development of virus-associated cancers. Additionally, the review highlights the growing interest among researchers regarding the implications of EXOs in cancer progression and their potential role in enhancing the oncogenicity of viruses.
Conclusion
The findings underscore the pivotal role of EXOs in mediating the oncogenic effects of viruses, suggesting that targeting exosomal pathways may provide new therapeutic avenues for managing virus-associated cancers. Further research is needed to fully elucidate the functional mechanisms of EXOs in viral oncogenesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


