Effect of bleaching powder (ClO−) on pulsating HGMS of chalcopyrite from arsenopyrite
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
High gradient magnetic separation (HGMS) is a potential method of separating chalcopyrite from copper-arsenopyrite co-flotation concentrate. However, during the co-flotation process the addition of bleaching powder (ClO−), which has been a common depressant for arsenopyrite, deteriorates their separation selectivity in the subsequent HGMS process, and until today this effect of ClO− is unknown to people. Pulsating HGMS (PHGMS) technology was used to separate a pure chalcopyrite-arsenopyrite mixture containing 16.85 % Cu and 22.44 % As. It was found that when the concentration of ClO− was increased from 0 % to 10 %, the Cu recovery in magnetic chalcopyrite concentrate was increased from 79.07 % to 88.69 %, with its Cu grade decreased from 20.25 % to 17.65 %; and, the As recovery in the concentrate was significantly increased from 26.82 % to 65.50 %, with As grade increased from 9.92 % to 18.83 %. The X-ray photoelectron spectroscopy (XPS) and electrochemical analysis on the chalcopyrite concentrate revealed that ClO− preferentially oxidized the iron sulfides in arsenopyrite to iron oxides; and, the Crystal Field Theory (CFT) analysis revealed that the magnetic moment of Fe in arsenopyrite was increased from 0 to 5.92B.M after these Fe atoms reacted with ClO−, and the number of single electrons in Fe was increased from 0 to 5. As a result, the magnetic susceptibility of arsenopyrite was increased and more arsenopyrite particles were separated into chalcopyrite concentrate during the PHGMS process, deteriorating the separation selectivity of chalcopyrite from arsenopyrite.
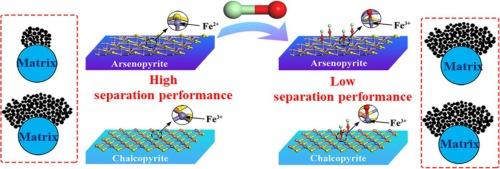
漂白粉(ClO-)对从砷黄铁矿中提取黄铜矿的脉动 HGMS 的影响
高梯度磁选(HGMS)是一种从铜砷黄铁矿共浮选精矿中分离黄铜矿的潜在方法。然而,在共浮选过程中,加入漂白粉(ClO-)会降低黄铜矿在随后的高梯度磁选过程中的分离选择性。我们利用脉动 HGMS(PHGMS)技术分离了含 16.85% 铜和 22.44% As 的纯黄铜矿-砷黄铁矿混合物。研究发现,当 ClO- 的浓度从 0 % 增加到 10 % 时,磁性黄铜矿精矿中的铜回收率从 79.07 % 增加到 88.69 %,铜品位从 20.25 % 降低到 17.65 %;精矿中的砷回收率从 26.82 % 显著增加到 65.50 %,砷品位从 9.92 % 增加到 18.83 %。对黄铜矿精矿进行的 X 射线光电子能谱(XPS)和电化学分析表明,ClO- 优先将砷黄铁矿中的硫化铁氧化成氧化铁;晶体场理论(CFT)分析表明,这些铁原子与 ClO-反应后,砷黄铁矿中铁的磁矩从 0 增加到 5.92B.M,铁中的单电子数从 0 增加到 5。因此,砷黄铁矿的磁感应强度增加,在 PHGMS 过程中,更多的砷黄铁矿颗粒被分离到黄铜矿精矿中,从而降低了黄铜矿与砷黄铁矿的分离选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


