Novel potent SOS1 inhibitors containing a tricyclic quinazoline scaffold: A joint view of experiments and simulations
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Small molecules that possess the ability to regulate the interactions between Son of Sevenless 1 (SOS1) and Kristen rat sarcoma (KRAS) offer immense potential in the realm of cancer therapy. In this study, we present a novel series of SOS1 inhibitors featuring a tricyclic quinazoline scaffold. Notably, we have identified compound 8d, which demonstrates the highest potency with an IC50 value of 5.1 nM for disrupting the KRAS:SOS1 interaction. Compound 8d exhibits a promising pharmacokinetic profile and achieves a remarkable 70.5 % inhibition of tumor growth in pancreas tumor xenograft models. Furthermore, molecular dynamic simulations have unveiled that the tricyclic quinazoline derivatives exhibit extensive interaction with Tyr884, a crucial residue for the recognition between SOS1 and KRAS. Our findings provide fresh insights into the design of future SOS1 inhibitors, paving the way for innovative therapeutic strategies.
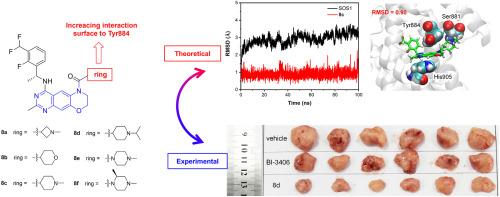
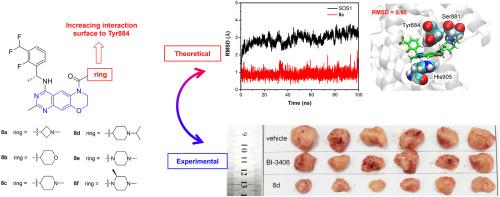
含有三环喹唑啉支架的新型强效 SOS1 抑制剂:实验与模拟的共同视角
具有调节七无之子 1(SOS1)和克里斯汀大鼠肉瘤(KRAS)之间相互作用能力的小分子在癌症治疗领域具有巨大的潜力。在这项研究中,我们提出了一系列新型 SOS1 抑制剂,它们具有三环喹唑啉支架。值得注意的是,我们发现了化合物 8d,它在破坏 KRAS:SOS1 相互作用方面表现出最高的效力,IC50 值为 5.1 nM。化合物 8d 具有良好的药代动力学特征,在胰腺肿瘤异种移植模型中对肿瘤生长的抑制率高达 70.5%。此外,分子动力学模拟揭示了三环喹唑啉衍生物与 Tyr884 的广泛相互作用,Tyr884 是 SOS1 与 KRAS 之间识别的关键残基。我们的发现为未来 SOS1 抑制剂的设计提供了新的见解,为创新治疗策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


