Discovery of a highly potent, N-terminal domain-targeting degrader of AR-FL/AR-V7 for the treatment of prostate cancer
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The clinical development of PROTACs targeting the androgen receptor (AR) for degradation has made significant progress. However, effective treatments for metastatic prostate cancers containing the androgen receptor splice variant 7 (AR-V7), a constitutively active mutant without the ligand-binding domain (LBD), are still lacking. Here, we reported the identification of a highly potent, noncovalent PROTAC targeting the N-terminal domain (NTD) of AR, NP18, which is developed from the covalent AR-NTD antagonist EPI-002, and effectively degrades both AR-FL and AR-V7 in 22Rv1 cells (DC50: 18 and 26 nM respectively). Mechanistically, NP18 interacts with the N-terminal domain (NTD) of both full-length AR (AR-FL) and splice variant 7 (AR-V7), leading to their selective and proteasomal degradation. Importantly, NP18 exhibited remarkably superior antitumor activity in both 22Rv1 xenograft and patient-derived xenograft (PDX) models than EPI-002. Taken together, these findings highlight NP18 as a promising candidate to counteract AR splice variant-driven resistance.
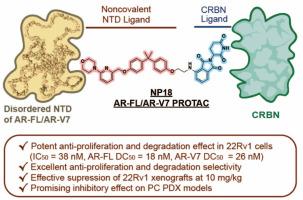
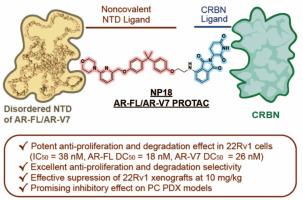
发现一种用于治疗前列腺癌的强效 N 端域靶向 AR-FL/AR-V7 降解剂
以降解雄激素受体(AR)为靶点的 PROTACs 临床开发取得了重大进展。然而,对于含有雄激素受体剪接变体7(AR-V7)(一种没有配体结合域(LBD)的组成性活性突变体)的转移性前列腺癌,仍然缺乏有效的治疗方法。在此,我们报告了一种靶向 AR N-末端结构域(NTD)的高效力非共价 PROTAC NP18 的鉴定结果,NP18 由共价 AR-NTD 拮抗剂 EPI-002 发展而来,能有效降解 22Rv1 细胞中的 AR-FL 和 AR-V7(DC50 分别为 18 和 26 nM)。从机理上讲,NP18 与全长 AR(AR-FL)和剪接变体 7(AR-V7)的 N 端结构域(NTD)相互作用,导致它们被蛋白酶体选择性降解。重要的是,与 EPI-002 相比,NP18 在 22Rv1 异种移植和患者来源异种移植 (PDX) 模型中均表现出明显更强的抗肿瘤活性。综上所述,这些发现凸显了NP18是一种有希望对抗AR剪接变体驱动的耐药性的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


