Deciphering the function of Fe3O4 in alleviating propionate inhibition during high-solids anaerobic digestion: Insights of physiological response and energy conservation
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
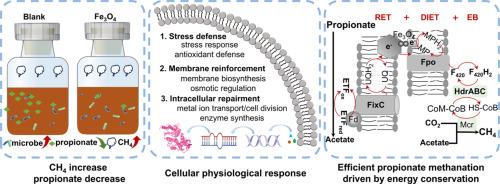
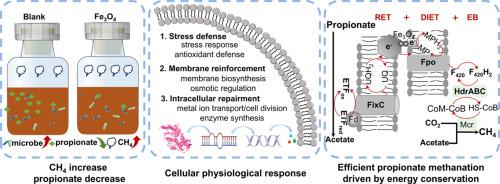
Fe3O4 is a recognized addictive to enhance low solid anaerobic digestion (AD), while for high solid AD challenged by acidity inhibition, its feasibility and mechanism remain unclear. In this study, the positive effect of Fe3O4 on high-solids AD of food waste by regulating microbial physiology and energy conservation to enhance mutualistic propionate methanation was documented. The methane yield was increased by 36.7 % with Fe3O4, which because Fe3O4 alleviated propionate stress on methane generation, along with improved propionate degradation and methanogenic metabolism. Fe3O4 facilitated the production of extracellular polymeric substances and the formation of tightly bio-aggregates, fostering an enriched microbial population (e.g., Smithella and Methanosaeta) to resist propionate stress. Also, Fe3O4 up-regulated the genes in stress defense system, cytomembrane biosynthesis/function, metal irons transporter, cell division and enzyme synthesis, verifying its superiority on cellular physiology. In addition, energy-conservation strategies related to intracellular and extracellular electron transfer were enhanced by Fe3O4. Specifically, the enzyme expressions involved in reversed electron transfer and electron bifurcation coupled with direct interspecies electron transfer (DIET) were upregulated by at least 2.2 times with Fe3O4, providing sufficient energy to drive thermodynamic adverse methanogenesis from propionate-stressed condition. Consequently, the reinforced enzyme expression in the dismutation and DIET pathway make it to be the predominant drivers for enhanced methanogenic propionate metabolism. This study fills the knowledge gaps of Fe3O4-induced microbial physiological and energetic strategies to resist environmental stress, and has remarkable practical implicated for restoring inhibited bioactivities.
解密 Fe3O4 在缓解高固体厌氧消化过程中丙酸盐抑制作用方面的功能:生理反应和能量守恒的启示
Fe3O4是一种公认的促进低固体厌氧消化(AD)的添加剂,但对于受到酸性抑制挑战的高固体厌氧消化,其可行性和机制仍不清楚。在这项研究中,Fe3O4 通过调节微生物生理机能和能量守恒来增强互生丙酸盐甲烷化,从而对食物垃圾的高固体厌氧消化产生了积极影响。使用 Fe3O4 后,甲烷产量增加了 36.7%,这是因为 Fe3O4 缓解了丙酸盐对甲烷生成的压力,同时改善了丙酸盐的降解和产甲烷代谢。Fe3O4 可促进胞外聚合物物质的产生和紧密生物聚集体的形成,促进微生物种群(如 Smithella 和 Methanosaeta)的丰富,从而抵御丙酸盐压力。此外,Fe3O4 还能上调应激防御系统、细胞膜生物合成/功能、金属铁转运、细胞分裂和酶合成等方面的基因,从而验证其在细胞生理方面的优势。此外,Fe3O4 还增强了与细胞内外电子传递相关的能量守恒策略。具体而言,参与反向电子传递和电子分叉以及种间直接电子传递(DIET)的酶表达量在Fe3O4的作用下至少提高了2.2倍,为丙酸盐胁迫条件下的热力学逆甲烷生成提供了充足的能量。因此,蜕变和 DIET 途径中酶表达的增强使其成为产甲烷丙酸盐代谢增强的主要驱动力。这项研究填补了有关氧化铁诱导微生物生理和能量策略以抵御环境压力的知识空白,对恢复被抑制的生物活性具有显著的实际意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


