Targeting the aminopeptidase ERAP enhances antitumor immunity by disrupting the NKG2A-HLA-E inhibitory checkpoint
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The aminopeptidase, endoplasmic reticulum aminopeptidase 1 (ERAP1), trims peptides for loading into major histocompatibility complex class I (MHC class I), and loss of this activity has broad effects on the MHC class I peptidome. Here, we investigated the impact of targeting ERAP1 in immune checkpoint blockade (ICB), as MHC class I interactions mediate both activating and inhibitory functions in antitumor immunity. Loss of ERAP sensitized mouse tumor models to ICB, and this sensitivity depended on CD8+ T cells and natural killer (NK) cells. In vivo suppression screens revealed that Erap1 deletion inactivated the inhibitory NKG2A-HLA-E checkpoint, which requires presentation of a restricted set of invariant epitopes (VL9) on HLA-E. Loss of ERAP altered the HLA-E peptidome, preventing NKG2A engagement. In humans, ERAP1 and ERAP2 showed functional redundancy for the processing and presentation of VL9, and loss of both inactivated the NKG2A checkpoint in cancer cells. Thus, loss of ERAP phenocopies the inhibition of the NKG2A-HLA-E pathway and represents an attractive approach to inhibit this critical checkpoint.
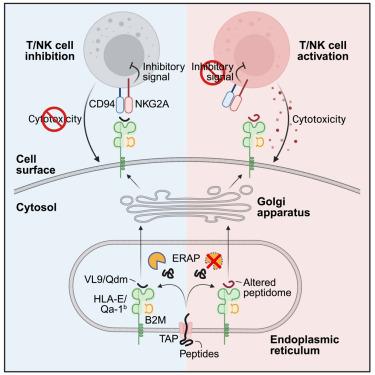
以氨基肽酶 ERAP 为靶点,通过破坏 NKG2A-HLA-E 抑制检查点增强抗肿瘤免疫力
氨肽酶--内质网氨肽酶 1(ERAP1)--修饰肽段以装入主要组织相容性复合体 I 类(MHC I 类),而这种活性的丧失会对 MHC I 类肽组产生广泛的影响。在这里,我们研究了靶向ERAP1对免疫检查点阻断(ICB)的影响,因为MHC I类相互作用在抗肿瘤免疫中同时介导激活和抑制功能。缺失ERAP会使小鼠肿瘤模型对ICB敏感,而这种敏感性取决于CD8+ T细胞和自然杀伤(NK)细胞。体内抑制筛选显示,Erap1缺失会使抑制性NKG2A-HLA-E检查点失活,而抑制性NKG2A-HLA-E检查点需要HLA-E上一组受限的不变表位(VL9)。ERAP的缺失改变了HLA-E肽组,阻止了NKG2A的参与。在人类中,ERAP1和ERAP2在处理和呈现VL9方面表现出功能冗余,两者的缺失会使癌细胞中的NKG2A检查点失活。因此,ERAP的缺失表征了对NKG2A-HLA-E通路的抑制,是抑制这一关键检查点的一种有吸引力的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


